Moxifloxacin hydrochloride sodium chloride injection and preparation method thereof
A technology of moxifloxacin hydrochloride and sodium chloride injection, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve product pollution, mismatching of content determination results, and influence on Product quality and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
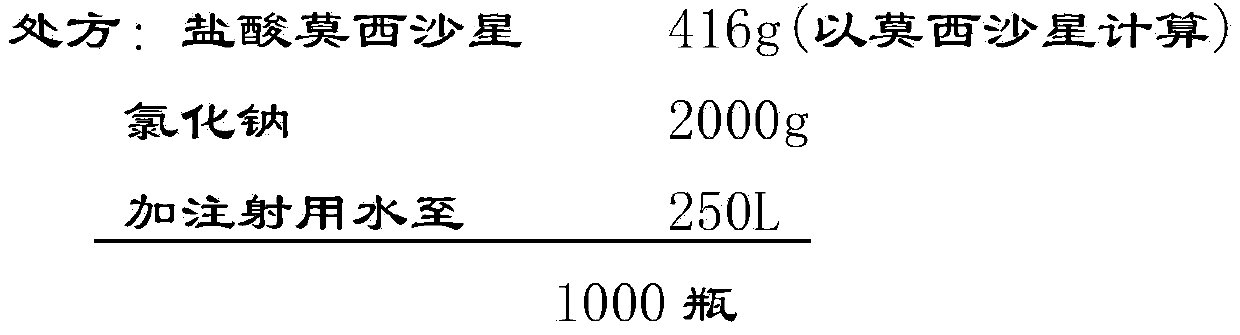
[0049] Implementation case 1: Preparation of Moxifloxacin Hydrochloride and Sodium Chloride Injection (250ml: 0.4g Moxifloxacin)
[0050] prescription:
[0051] (1) Weigh the qualified moxifloxacin hydrochloride raw materials and sodium chloride according to the prescription;
[0052] (2) Add 70% of the total volume of injection water to the batching tank and cool to 45~55℃;
[0053] (3) Add the moxifloxacin hydrochloride raw material weighed according to the prescription and stir to make it fully dissolved;
[0054] (4) Put in sodium chloride in the batching amount and stir until it is completely dissolved;
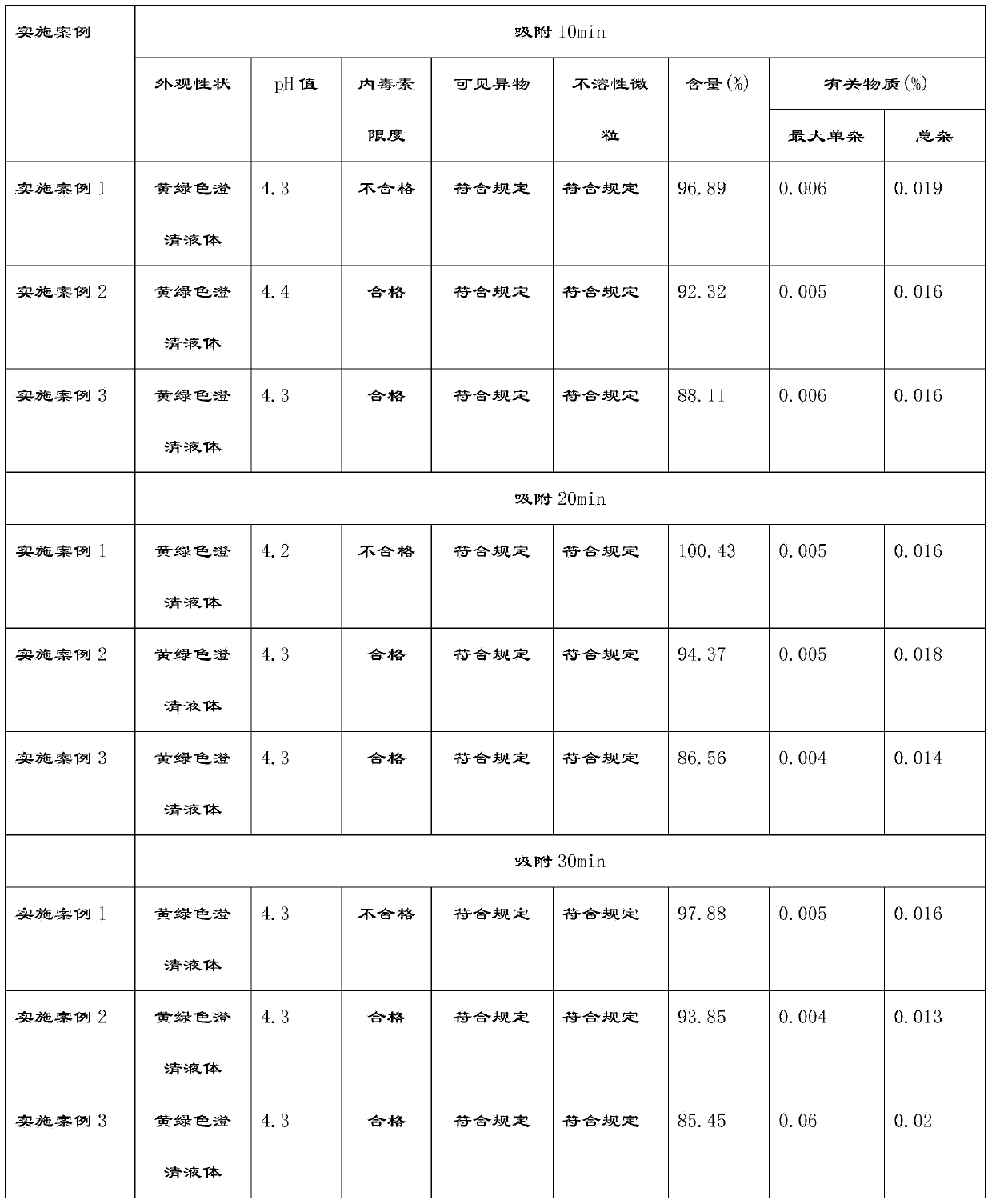
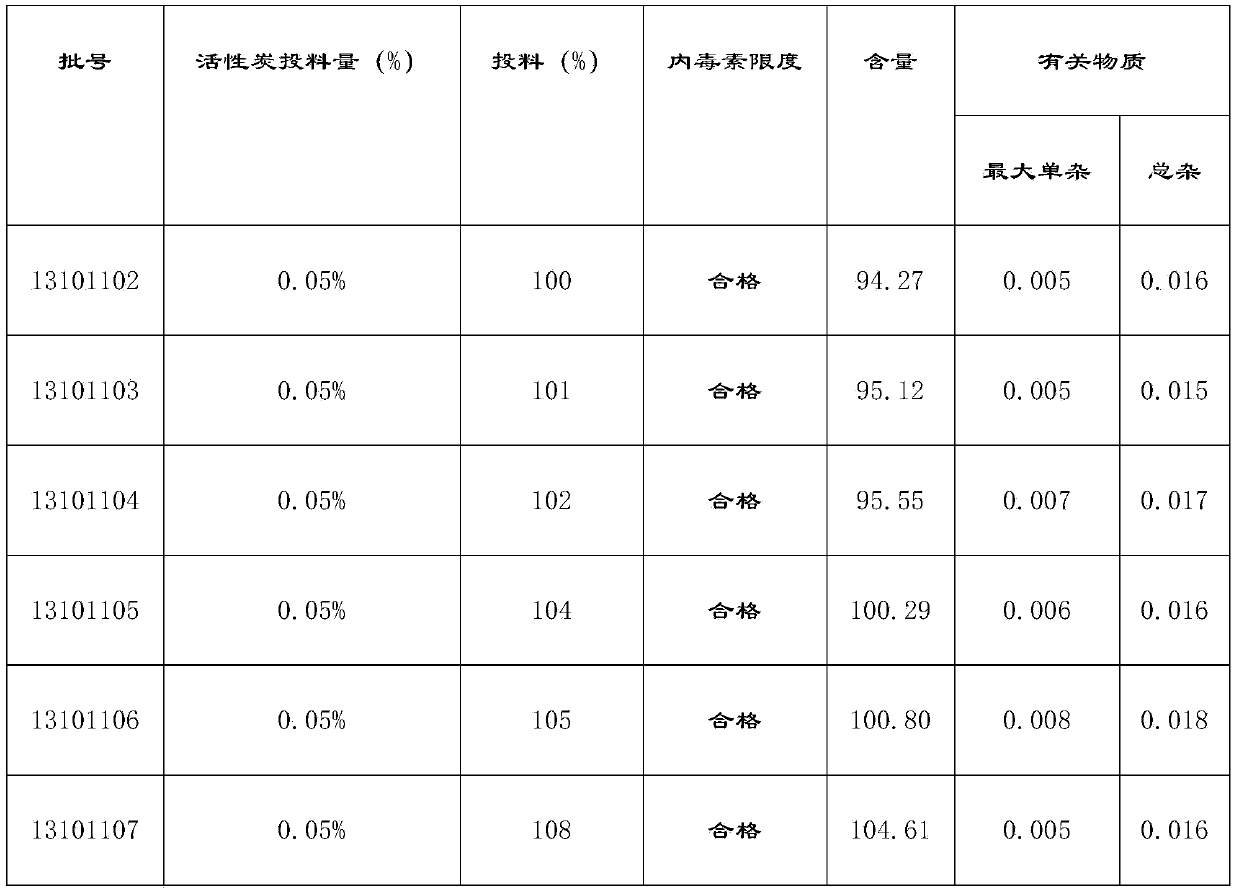
[0055] (5) Add activated carbon for injection at 0.025% (w / v) of the volume of the concentrated solution, stir for 10-30 minutes at 45-55°C, filter back for 5-10 minutes, pass through the titanium rod filter with a pump A set of 0.45μm cartridge type microporous filter membrane is hit to the rare distribution tank;
[0056] (6) Add water for injection to the batch mark. After stir...
Embodiment example 2
[0061] Implementation case 2: Preparation of Moxifloxacin Hydrochloride and Sodium Chloride Injection (250ml: 0.4g Moxifloxacin)
[0062] prescription:
[0063] (1) Weigh the qualified moxifloxacin hydrochloride raw materials and sodium chloride according to the prescription;
[0064] (2) Add 70% of the total volume of injection water to the batching tank and cool to 45~55℃;
[0065] (3) Add the moxifloxacin hydrochloride raw material weighed according to the prescription and stir to make it fully dissolved;
[0066] (4) Put in sodium chloride in the batching amount and stir until it is completely dissolved;
[0067] (5) Add activated carbon for injection according to 0.05% (w / v) of the volume of the concentrated solution, stir for 10-30 minutes at 45-55°C, filter back for 5-10 minutes, pass through the titanium rod filter with a pump A set of 0.45μm cartridge type microporous filter membrane is hit to the rare distribution tank;
[0068] (6) Add water for injection to the batch mark. A...
Embodiment example 3
[0073] Implementation case 3: Preparation of Moxifloxacin Hydrochloride and Sodium Chloride Injection (250ml: 0.4g Moxifloxacin)
[0074] prescription:
[0075] (1) Weigh the qualified moxifloxacin hydrochloride raw materials and sodium chloride according to the prescription;
[0076] (2) Add 70% of the total volume of injection water to the batching tank and cool to 45~55℃;
[0077] (3) Add the moxifloxacin hydrochloride raw material weighed according to the prescription and stir to make it fully dissolved;
[0078] (4) Put in sodium chloride in the batching amount and stir until it is completely dissolved;
[0079] (5) Add activated carbon for injection according to 0.1% (w / v) of the volume of the concentrated solution, stir for 10-30 minutes at 45-55°C, filter back for 5-10 minutes, pass through the titanium rod filter with a pump A set of 0.45μm cartridge type microporous filter membrane is hit to the rare distribution tank;
[0080] (6) Add water for injection to the batch mark. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com