Moxifloxacinhydrochloride sodium chloride injection, preparation method thereof and use thereof
A technology of moxifloxacin hydrochloride and sodium chloride injection, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
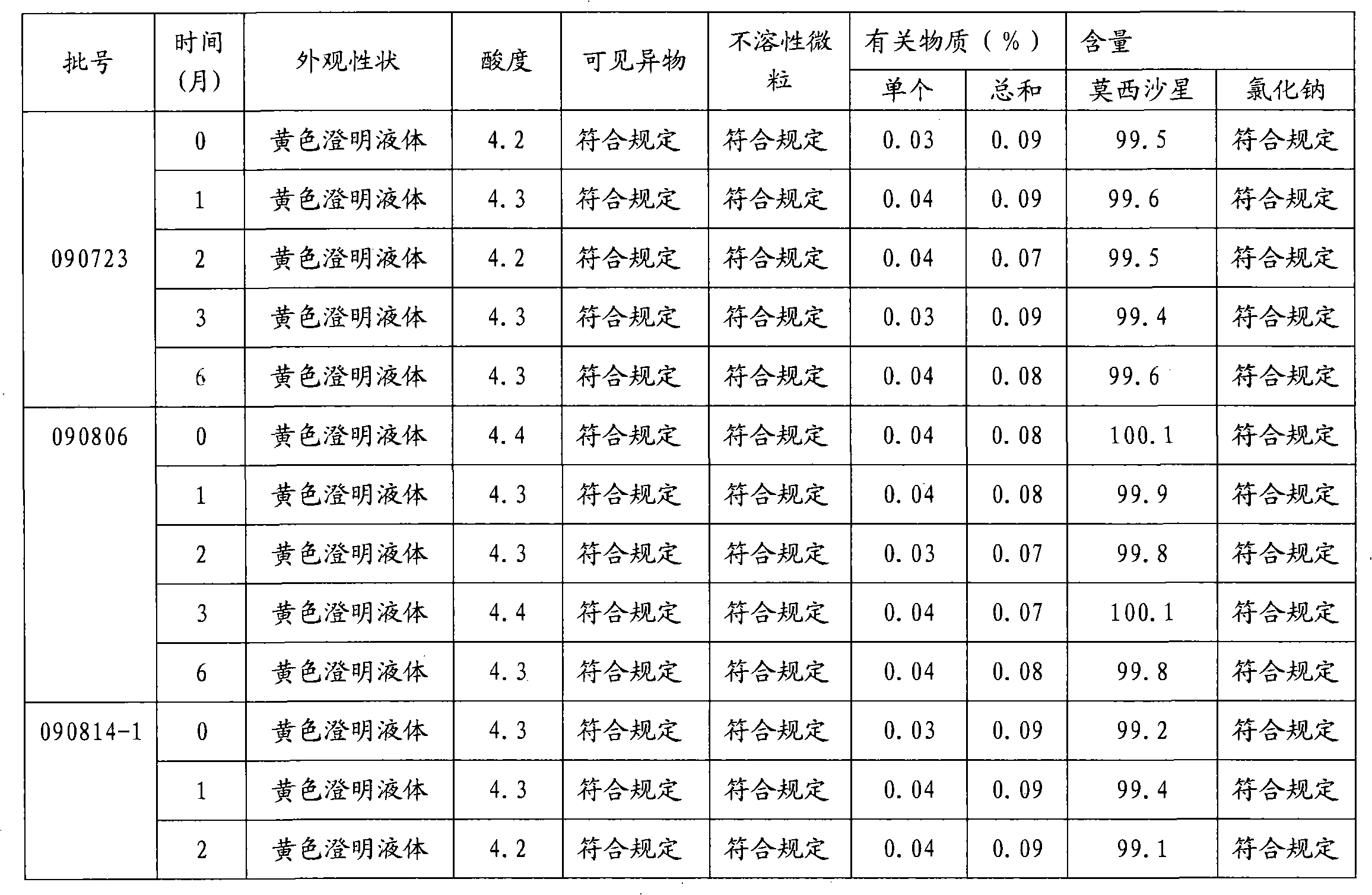
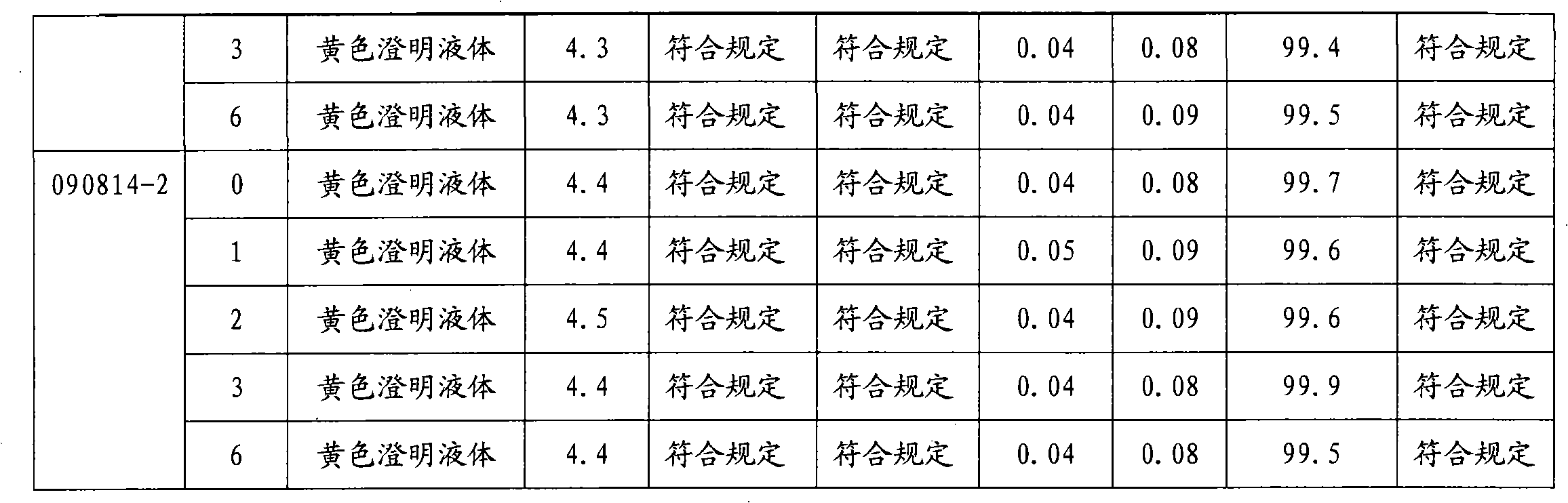
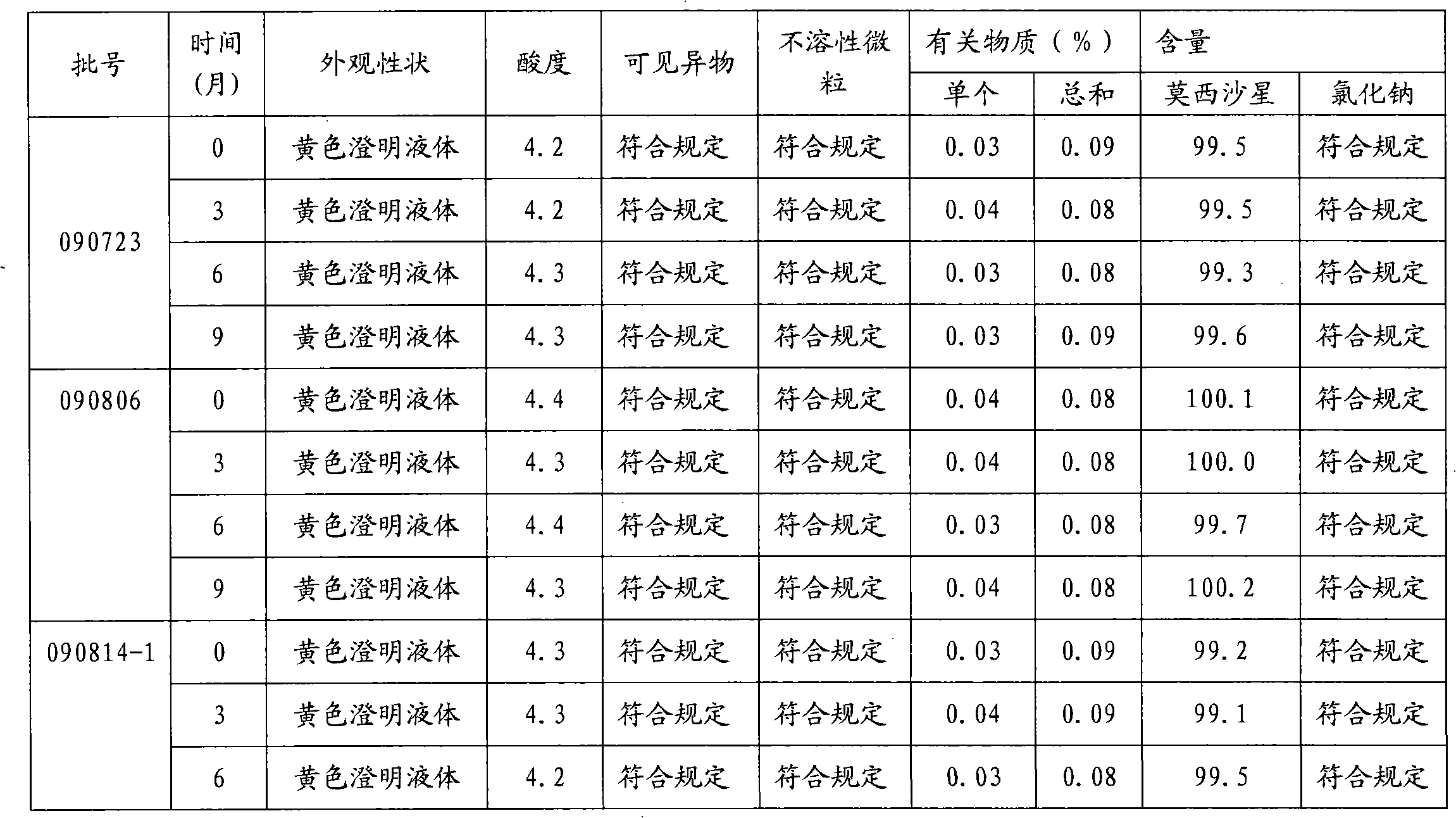
[0018] Embodiment 1: the preparation of moxifloxacin hydrochloride sodium chloride injection (0.4g / 250ml)
[0019] Prescription: Moxifloxacin Hydrochloride 400g (calculated as Moxifloxacin)
[0020] Sodium chloride 2.0kg
[0021] Sodium acetate 250g
[0022] Add water for injection to 250L
[0023]
[0024] 1000 bottles
[0025] 1) Obtain qualified moxifloxacin hydrochloride raw materials, sodium chloride and sodium acetate according to the ingredient list;
[0026] 2) Add water for injection with a batch volume of 95% in the batching tank, add sodium acetate and moxifloxacin hydrochloride in the batching amount, stir to fully dissolve, add sodium chloride in the prescribed amount, adjust the pH value to 4.4 with acetic acid, and adjust the pH value to 4.4 with acetic acid. Add 0.05% of the volume (W / V) to medicinal charcoal and stir evenly, keep warm at 70°C to 80°C for 20 minute...
Embodiment 2
[0033] Embodiment 2: the preparation of moxifloxacin hydrochloride sodium chloride injection (0.4g / 100ml)
[0034] Prescription: Moxifloxacin Hydrochloride 400g (calculated as Moxifloxacin)
[0035] Sodium chloride 800g
[0036] Sodium citrate 100g
[0037] Add water for injection to 100L
[0038]
[0039] 1000 bottles
[0040] 1) Obtain qualified moxifloxacin hydrochloride raw materials, sodium chloride and sodium citrate according to the ingredient list;
[0041]2) Add water for injection with a batch volume of 90% in the batching tank, add the batching amount, sodium citrate and moxifloxacin hydrochloride, stir to fully dissolve, add the prescribed amount of sodium chloride and stir to dissolve, adjust with citric acid The pH value is 4.0-4.5, add medicinal charcoal according to 0.05% (W / V) of the total volume, stir evenly, keep warm at 70°C-80°C for 20 minutes, and circulate...
Embodiment 3
[0048] Embodiment 3: the preparation of moxifloxacin hydrochloride sodium chloride injection (0.4g / 250ml)
[0049] Prescription: Moxifloxacin Hydrochloride 400g (calculated as Moxifloxacin)
[0050] Sodium chloride 2kg
[0051] Sodium citrate 250g
[0052] Add water for injection to 250L
[0053]
[0054] 1000 bottles
[0055] 1) Obtain qualified moxifloxacin hydrochloride raw materials, sodium chloride and sodium citrate according to the ingredient list;
[0056] 2) Add water for injection with a batch volume of 80% in the batching tank, add the batching amount, sodium citrate and moxifloxacin hydrochloride, stir to fully dissolve, add the prescribed amount of sodium chloride and stir to dissolve, adjust with citric acid The pH value is 4.0-4.5, add medicinal charcoal according to 0.05% (W / V) of the total volume, stir evenly, keep warm at 70°C-80°C for 20 minutes, and circulate and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com