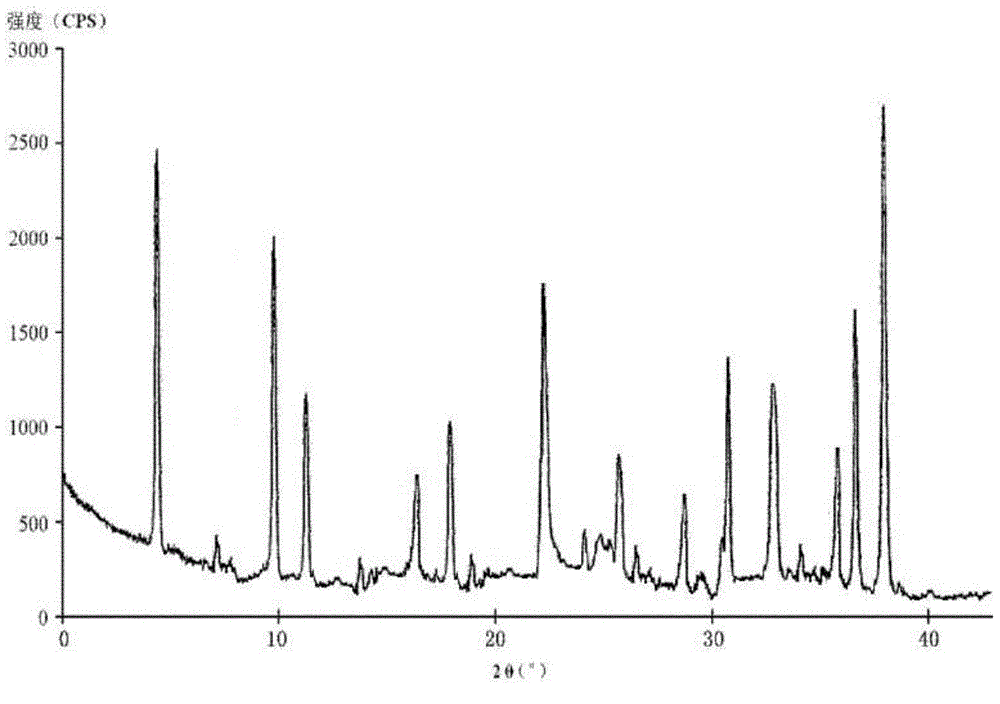
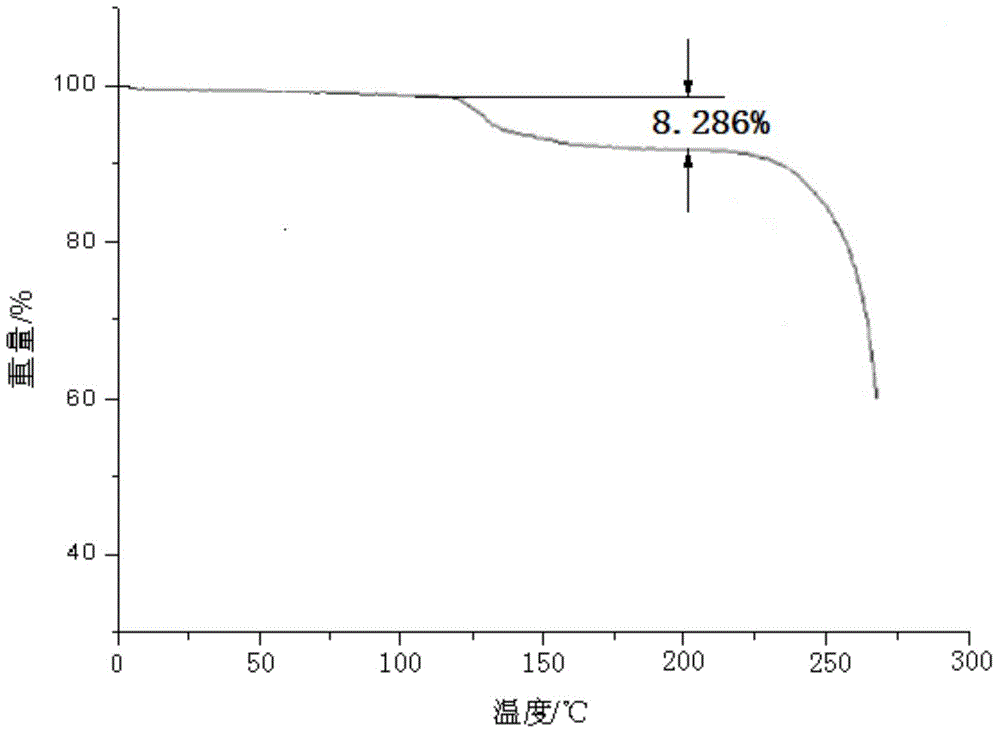
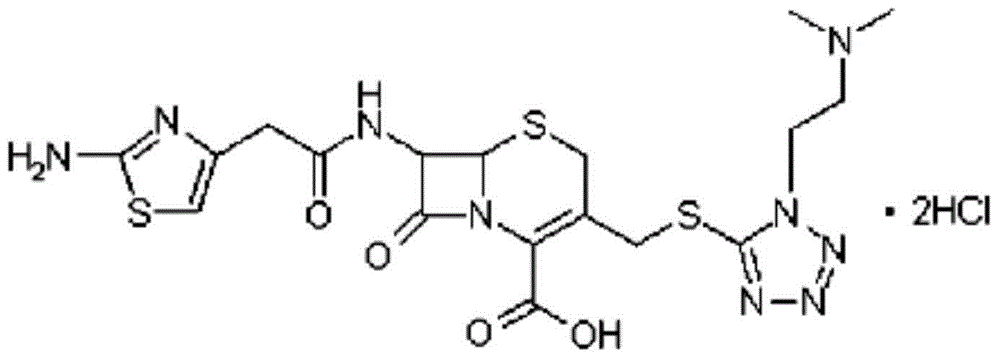
Cefotiam hydrochloride compound, method for preparing same and pharmaceutical composition with cefotiam hydrochloride compound
A technology of cefotiam hydrochloride and cefotiam hydrochloride, applied in the field of medicine, can solve the problems of poor mixing uniformity, poor fluidity of cefotiam hydrochloride, unstable cefotiam hydrochloride for injection, etc., and achieves good fluidity and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Preparation of Cefotiam Hydrochloride Compound
[0047] [Example 1] Preparation of Cefotiam Hydrochloride Compound
[0048] 1) Dissolve 12kg crude cefotiam hydrochloride in 10L water to obtain 1.2kg / L cefotiam hydrochloride aqueous solution;
[0049] 2) Add activated carbon to the cefotiam hydrochloride aqueous solution of step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution), stir, filter with suction, and take the filtrate;
[0050] 3) Add 50L of organic solvent A (the flow rate of organic solvent A is 13L / min) to the filtrate of step 2) at a stirring speed of 50r / min to form a turbid solution, wherein the organic solvent A is ethanol A mixed solvent composed of dioxane and dioxane in a volume ratio of 2:1;
[0051] 4) After adjusting the stirring speed to 15r / min, add 300L of organic solvent B (the flow acceleration of organic solvent B is 12L / min) into the turbid solution of step 3). After the addition, ...
Embodiment 2
[0057] [Example 2] Preparation of Cefotiam Hydrochloride Compound
[0058] 1) Dissolve 22kg of crude cefotiam hydrochloride in 10L of water to obtain 2.2kg / L cefotiam hydrochloride aqueous solution;
[0059] 2) Add activated carbon to the cefotiam hydrochloride aqueous solution of step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution), stir, filter with suction, and take the filtrate;
[0060] 3) Add 100L of organic solvent A (the flow rate of organic solvent A is 20L / min) to the filtrate of step 2) at a stirring speed of 60r / min to form a turbid solution, wherein the organic solvent A is ethanol A mixed solvent composed of dioxane and dioxane in a volume ratio of 3:1;
[0061] 4) After adjusting the stirring speed to 25r / min, add 200L of organic solvent B (the flow rate of organic solvent B is 6L / min) to the turbid solution of step 3). After the addition, crystals are precipitated. The organic solvent B It is a mixed solven...
Embodiment 3
[0066] [Example 3] Preparation of Cefotiam Hydrochloride Compound
[0067] 1) Dissolve 20kg crude cefotiam hydrochloride in 10L water to obtain 2.0kg / L cefotiam hydrochloride aqueous solution;
[0068] 2) Add activated carbon to the cefotiam hydrochloride aqueous solution of step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution), stir, filter with suction, and take the filtrate;
[0069] 3) Add 80L of organic solvent A (the flow rate of organic solvent A is 15L / min) to the filtrate of step 2) at a stirring speed of 55r / min to form a turbid solution, wherein the organic solvent A is ethanol A mixed solvent composed of 2.5:1 by volume with dioxane;
[0070] 4) After adjusting the stirring speed to 20r / min, add 100L of organic solvent B to the turbid solution of step 3) (the flow rate of organic solvent B is 8L / min), after the addition, crystals are precipitated, wherein the organic solvent B It is a mixed solvent composed of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com