Children type azlocillin sodium and low-sodium carrier pharmaceutical composition
A technology of azlocillin sodium and composition, applied in the field of children's azlocillin sodium and low-sodium carrier pharmaceutical composition, can solve the problems of occurrence of degradation products, easy discoloration of appearance, poor stability, etc., to simplify steps and improve clinical performance. Good application quality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
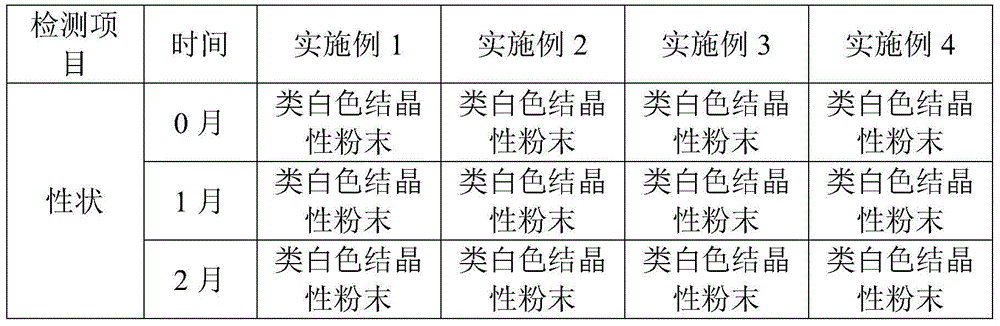
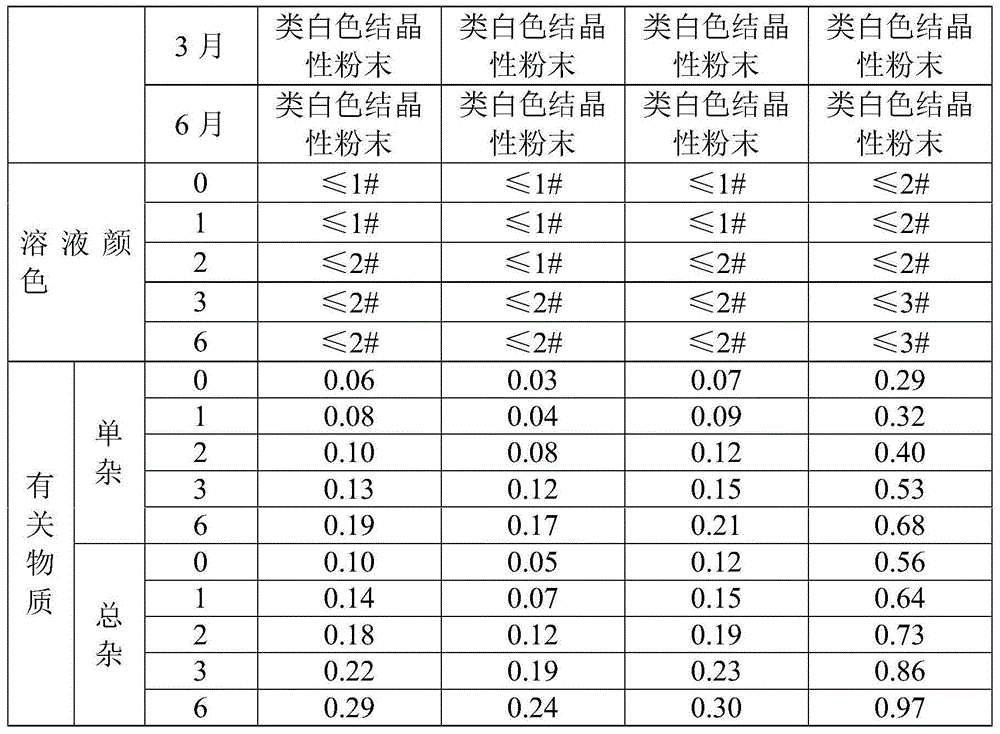
Examples
Embodiment 1
[0043] Add 400ml of acetone, 80ml of water and 100g of azlocillinic acid into the reactor, stir at 25°C until dissolved, add dropwise 100ml of 0.2g / ml anhydrous sodium acetate ethanol solution within 30 minutes while stirring; continue stirring for 30min, and grow crystals , filtered with suction, washed with acetone, and dried under vacuum at 40°C to obtain crude azlocillin sodium.
[0044] Weigh 100 g of the crude product of azlocillin sodium, add 1000 ml of purified water, raise the temperature to 30°C until completely dissolved, add 10 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0045] Add 5ml of ethyl acetate to the above filtrate under stirring, transfer to a 1000ml pressure-resistant container, make sure it is full and the air bubbles are removed, seal the container, oscillate, freeze at -20°C for 2 hours, and take it out.
[0046] Separate the liquid from the solid, discard the ethyl acetate solution, transfer to a crystallization ta...
Embodiment 2
[0048] Add 300ml of acetone, 100ml of water and 100g of azlocillinic acid into the reactor, stir at a temperature of 25°C until dissolved, add 110ml of 0.2g / ml anhydrous sodium acetate ethanol solution dropwise within 30 minutes while stirring; continue stirring for 30min, and grow crystals , filtered with suction, washed with acetone, and dried under vacuum at 45°C to obtain crude azlocillin sodium.
[0049] Weigh 100 g of crude azlocillin sodium, add 500 ml of purified water, heat up to 30°C until completely dissolved, add 7 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0050] Add 5ml of chloroform to the above filtrate under stirring, transfer it to a 500ml pressure-resistant container, make sure it is full and the air bubbles are removed, seal the container, oscillate, freeze at -15°C for 5 hours, and take it out.
[0051]Separate the liquid from the solid, discard the chloroform solution, transfer to the crystallization tank after the ice...
Embodiment 3
[0053] Add 200ml of acetone, 150ml of water and 100g of azlocillinic acid into the reactor, stir at 25°C until dissolved, add 124ml of 0.2g / ml anhydrous sodium acetate ethanol solution dropwise within 30 minutes while stirring; continue stirring for 30min, and grow crystals , filtered with suction, washed with acetone, and dried under vacuum at 50°C to obtain crude azlocillin sodium.
[0054] Weigh 100 g of the crude product of azlocillin sodium, add 350 ml of purified water, raise the temperature to 30°C until completely dissolved, add 3 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0055] Add 2.3ml of ethyl acetate and 2.3ml of chloroform mixed solvent to the above filtrate under stirring, transfer to a 350ml pressure-resistant container, make sure it is full and air bubbles are removed, seal the container, oscillate, freeze at -10°C for 8 hours, and then take it out.
[0056] Separate the liquid from the solid, and discard the mixed solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com