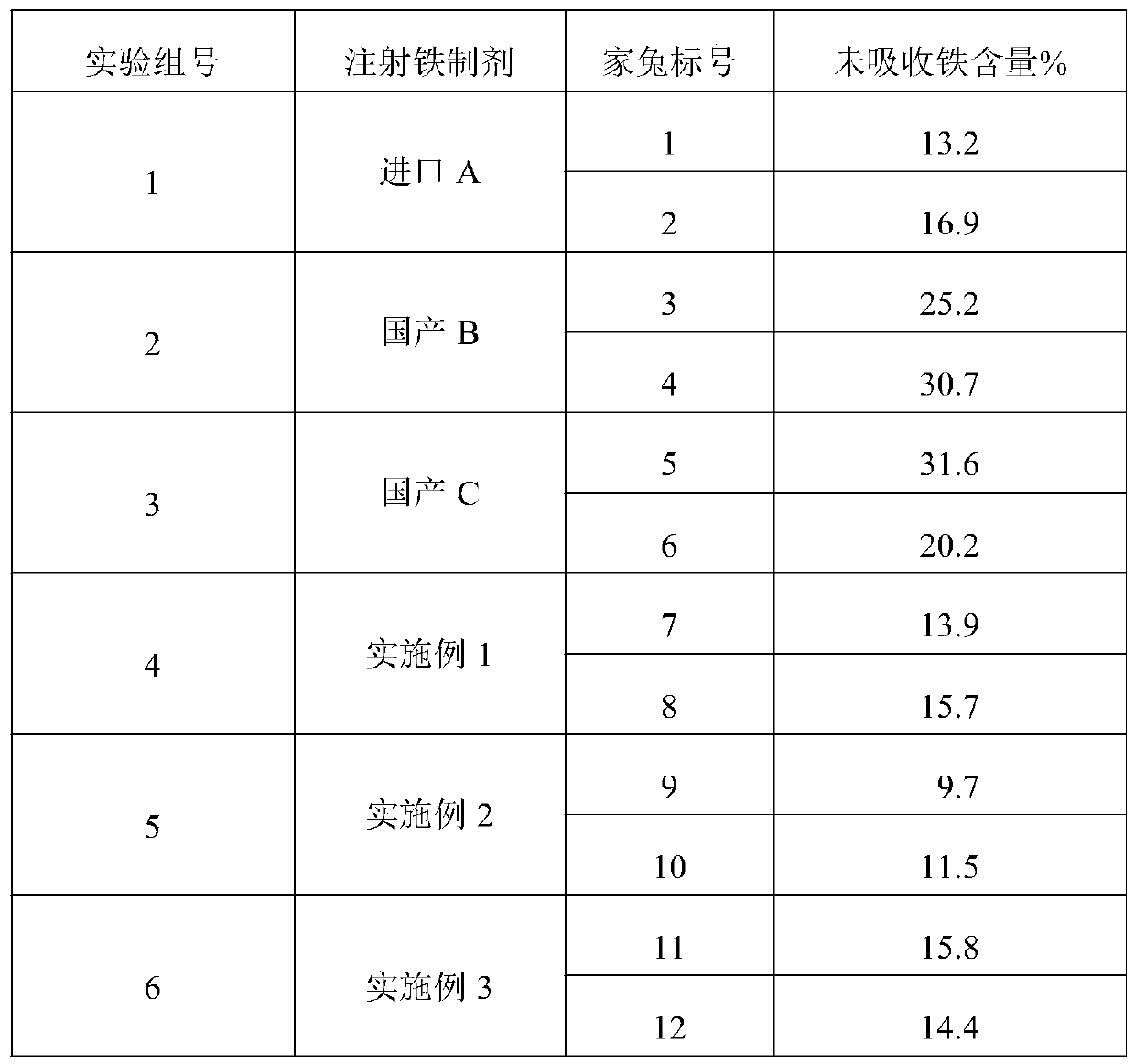
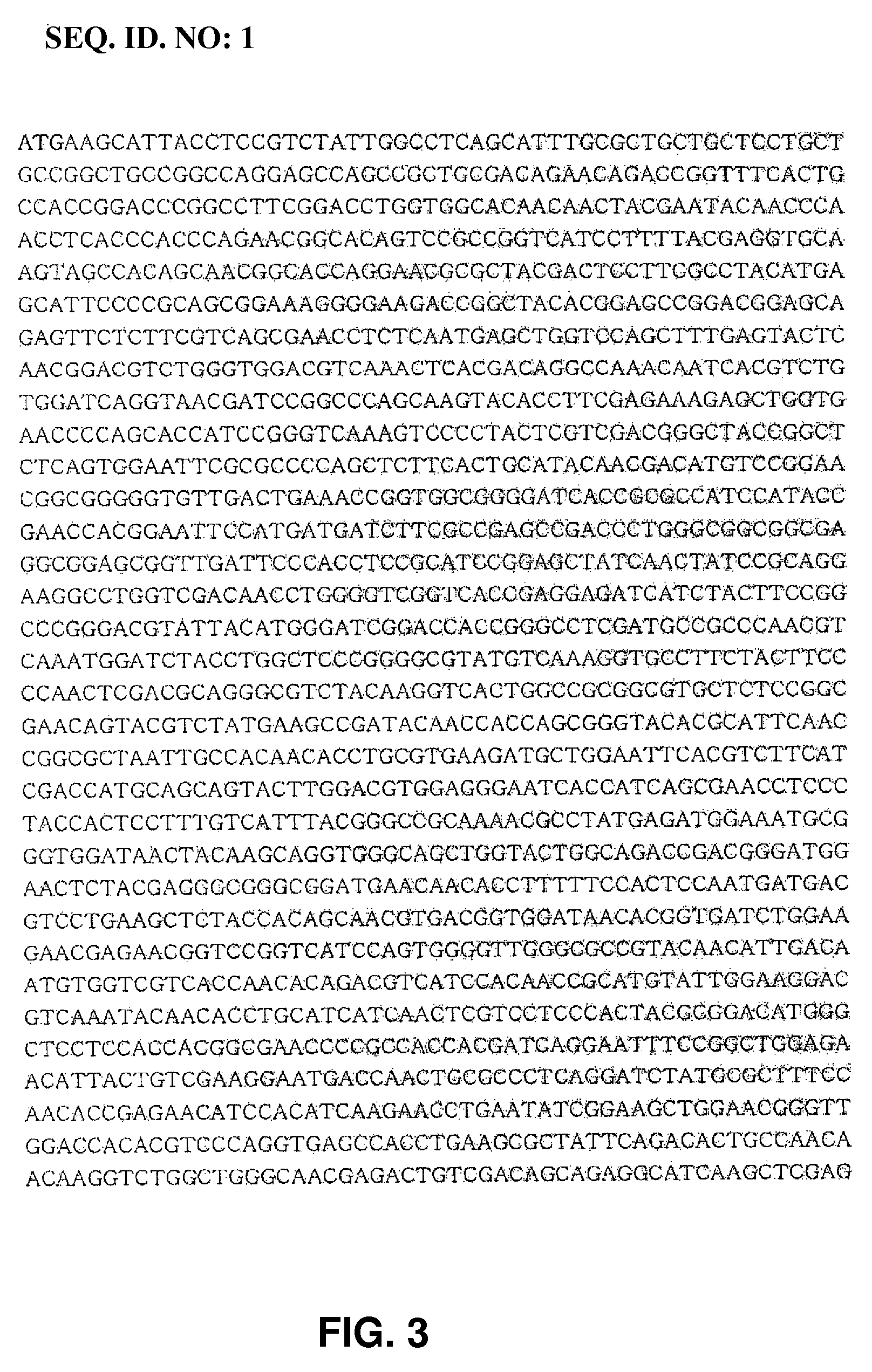Patents
Literature
35 results about "Low molecular weight dextran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Native dextran is a polymer of glucose with a molecular weight around 40 million; from which is prepared relatively lower molecular weight dextrans: high molecular weight (HMW) dextran of molecular weight 70000 and Low molecular weight (LMW) dextran, which is a mixture of dextrose polymers with an average molecular weight of 40000 (i.e., 10000-70000 ...
Molded object comprising alpha-1,4-glucans and/or modifications thereof and process for producing the same
InactiveUS20060134417A1Improve gelationPromote degradationSugar derivativesFibre chemical featuresPolymer scienceGlucan
The present invention discloses a molded article containing high molecular weight α-1,4-glucan and / or its modification, and low molecular weight α-1,4-glucan and / or its modification. The low molecular weight α-1,4-glucan has the degree of polymerization of greater than or equal to 180 and less than 620. And the high molecular weight α-1,4-glucan has the degree of polymerization of greater than or equal to 620 and less than 37000. The high molecular weight α-1,4-glucan may preferably have a molecular weight distribution of not greater than 1.25, and the low molecular weight α-1,4-glucan may preferably have a molecular weight distribution of not greater than 1.25. The present invention also discloses a process for preparing the molded article, wherein the process comprises the step of adding the low molecular weight α-1,4-glucan and / or its modification to a solution comprising the high molecular weight α-1,4-glucan and / or its modification to gel the solution.
Owner:EZAKI GLICO CO LTD +1
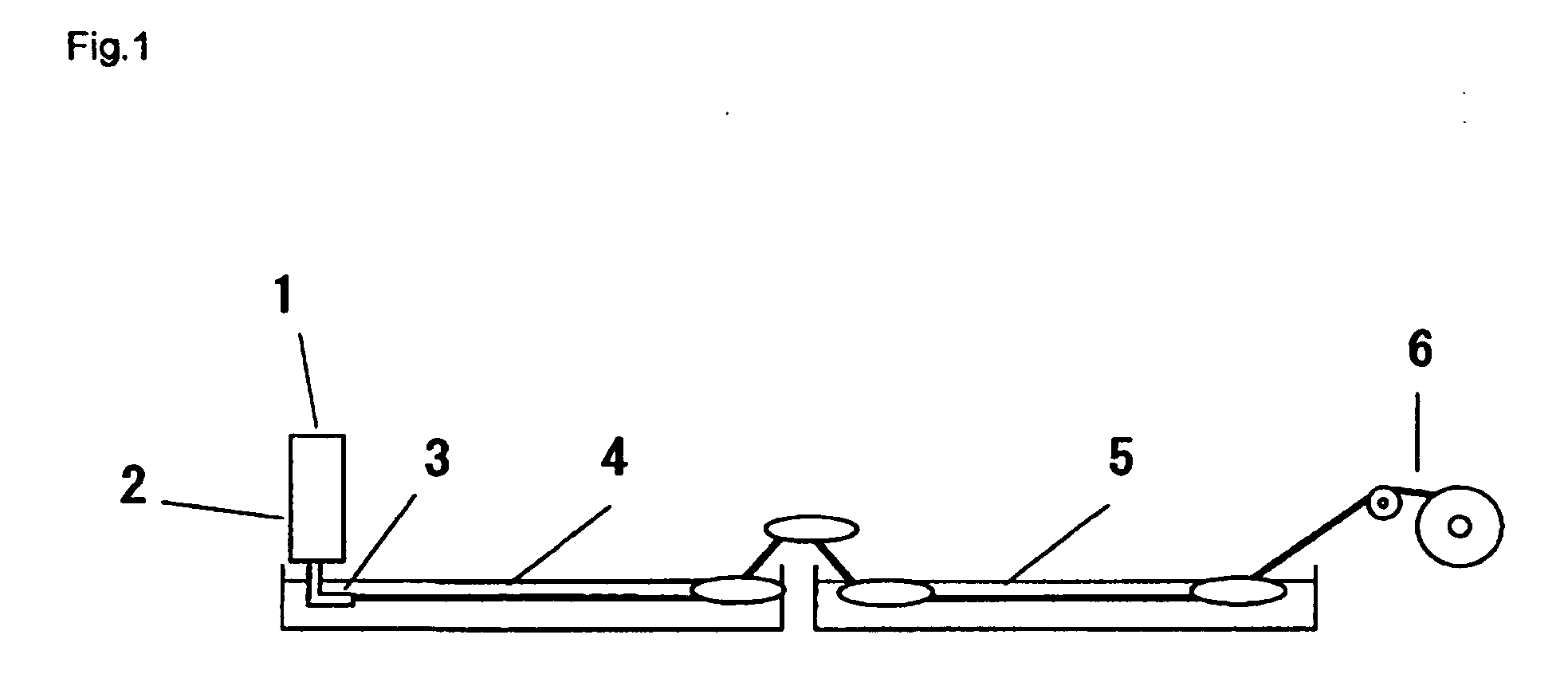
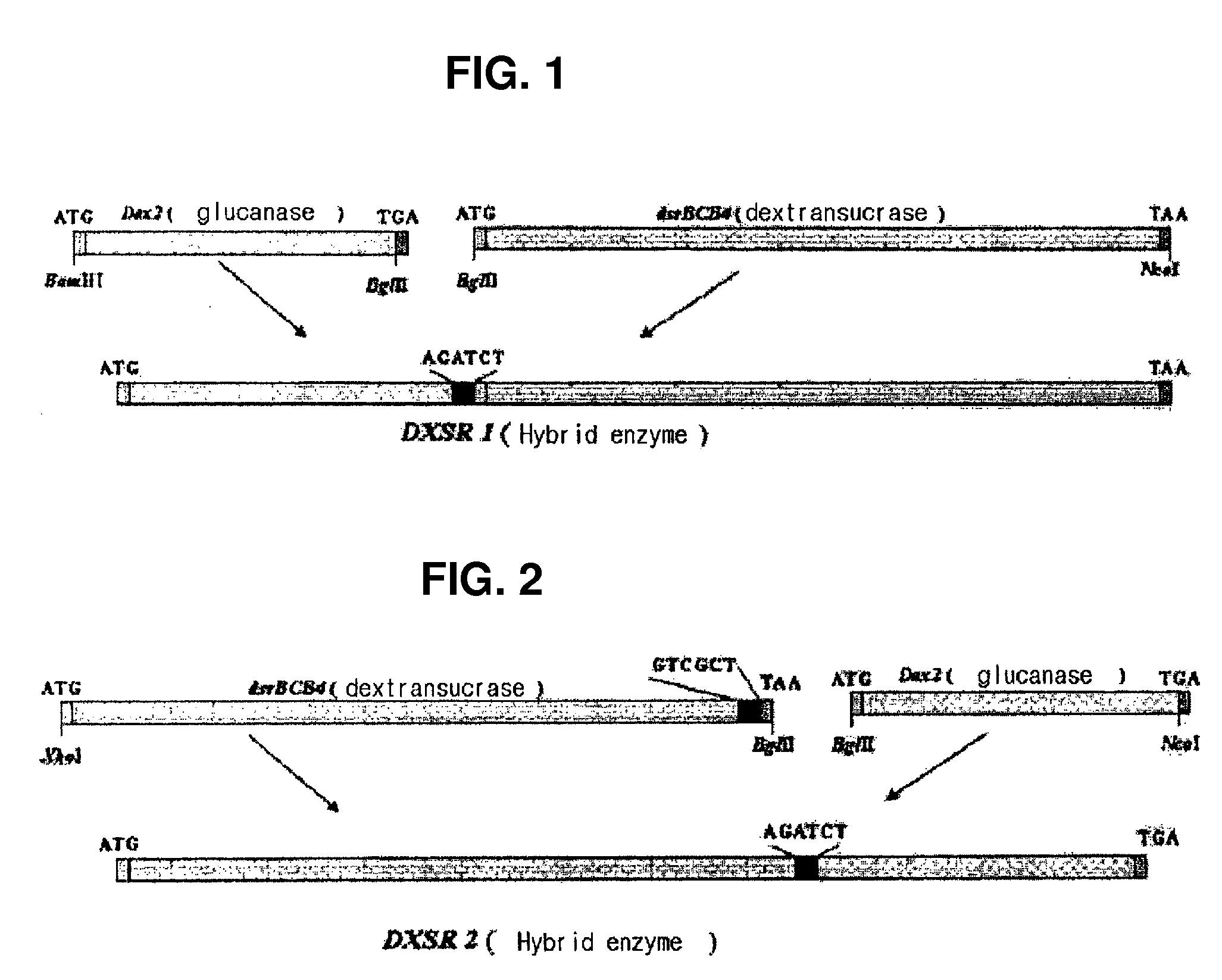
Hybrid genes and enzymes of glucanase and dextransucrase and processes for preparing isomalto-oligosaccharides or dextran using the same
Owner:IND FOUND OF CHONNAM NAT UNIV
Preparation method and use of low-molecular weight beta-glucan
The invention discloses a preparation method and a use of low-molecular weight beta-glucan and belongs to the technical field of food and beverage processing. The preparation method comprises the following steps of preparing an aqueous solution having a mass concentration of 1-5% from marketed food-grade cereal beta-glucan, carrying out treatment by endo-type beta-glucanase for 0.5-4h, controlling viscosity (2%, <100cp) and molecular weight (of 10-100KDa), carrying out decoloring by active carbon, collecting beta-glucan having the molecular weight of 10-100KDa by an ultrafilter membrane, and carrying out freeze drying to obtain a product. The low-molecular weight beta-glucan can be used in foods such as beer and beverages, improves daily intake of beta-glucan, improves human immunity and reduces cholesterol.
Owner:JIANGNAN UNIV
Method for preparing medium-low molecular weight dextran by using biological method
ActiveCN102690857ARealize regulationHigh yieldOrganic active ingredientsCosmetic preparationsLeuconostoc mesenteroidesMicroorganism
The invention discloses a method for preparing medium-low molecular weight dextran by using a biological method. The method comprises the following steps: adding a leuconostoc mesenteroides seed solution in a fermentation medium containing sucrose for carrying out fermentation culture; then adding a penicillium cyclopium seed solution or fermentation broth supernate of the penicillium cyclopium for continuously fermenting and culturing; and separating dextran from the fermentation broth to obtain the medium-low molecular weight dextran. The invention further discloses the prepared dextran and application thereof. The invention provides a method for directly obtaining the medium-low molecular weight dextran by using the biological method in a mode of coupling fermentation of double microorganisms. The medium-low molecular weight dextran is synthesized by directionally regulating and controlling through the biological method, so that the generated dextran degrades to form dextran which tends to be required by clinical application. Meanwhile, according to the method disclosed by the invention, ethanol precipitation can be directly carried out, and thus redundant, time-consuming and noble gradual dividing precipitation is omitted, the yield of the dextran is increased, and the use amount and the consumption of ethanol are reduced.
Owner:GUANGXI UNIV FOR NATITIES
Preparation method of adenosine triphosphate coenzyme insulin freeze-dried powder injection
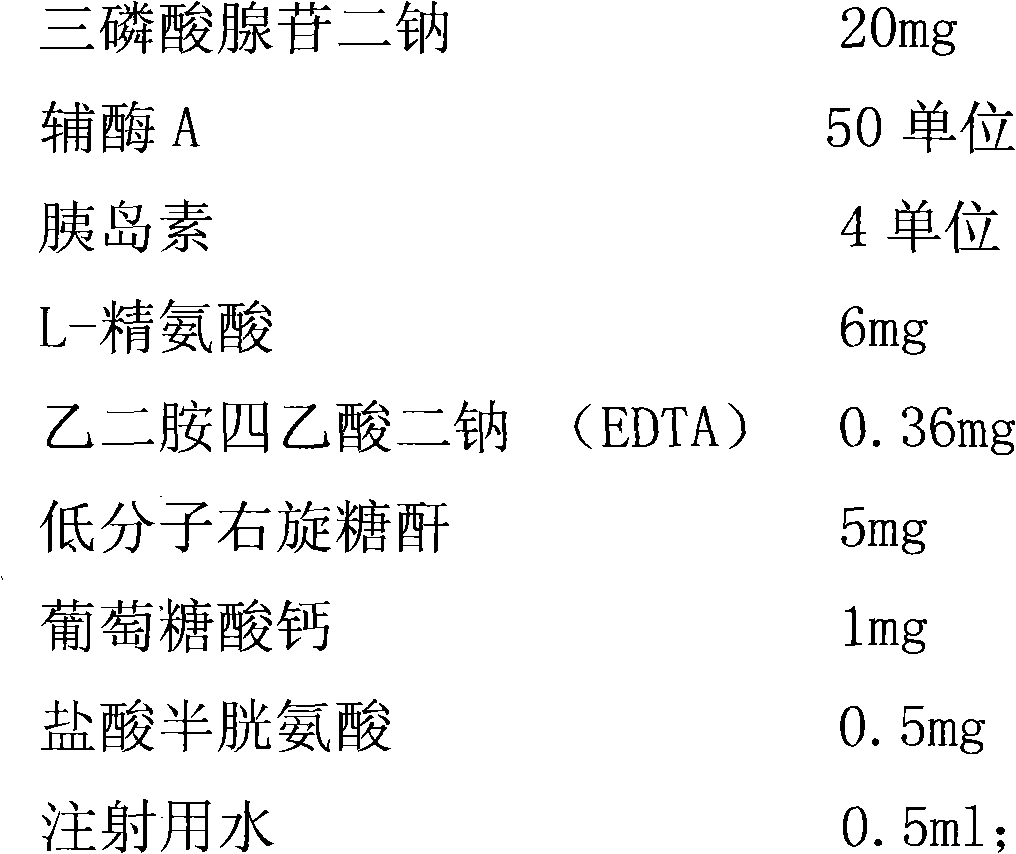
The invention discloses a preparation method of an adenosine triphosphate coenzyme insulin freeze-dried powder injection, comprising the following steps of: adjusting injection water with diluted hydrochloric acid until the pH value is 2.5-3.5, adding insulin and uniformly mixing to obtain a solution I; adding L-arginine and disodium ethylene diamine tetraacetate in the injection water, uniformlystirring, adding carbon and stirring; after removing carbon, adding adenosine disodium triphosphate and uniformly stirring to obtain a solution II; adding calcium gluconate, low molecular weight dextran and cysteine hydrochloride in the injection water, adding carbon and removing the carbon and uniformly stirring to obtain a solution III; mixing three solutions, uniformly stirring, adjusting the pH value to be 5.7-6.2 with a 2-4 percent NaOH solution, adding coenzyme A and supplementing the balance of injection water; and sterilizing, measuring the content, subpackaging and freezing dry. The adenosine triphosphate coenzyme insulin freeze-dried powder injection prepared by the method has the advantages of favorable redissolution, clearness and quality stability of products and is saturatedin appearance.
Owner:NORTH CHINA PHARMA COMPANY
Troxerutin powder injection
InactiveCN1586467AImprove stabilityQuality assurancePowder deliveryOrganic active ingredientsHydrolysateFreeze-drying
The troxerutin powder for injection includes troxerutin 2-100 wt% and excipient 0-98 wt%, and the excipient is one of mannitol, lactose, glycine, sorbic alcohol, low molecular weight dextran, glucose, cane sugar and hydrolysate gel. It is bacteria-free powder or bacteria-free freeze dried produce. The present invention is used in treating ischemic cerebral vascular diseases, central retinitis, arteriosclerosis, thrombotic phlebitis, varicosity, etc. and has the advantages of stability, fast dissolution in water and high safety.
Owner:广东阳江制药厂有限公司
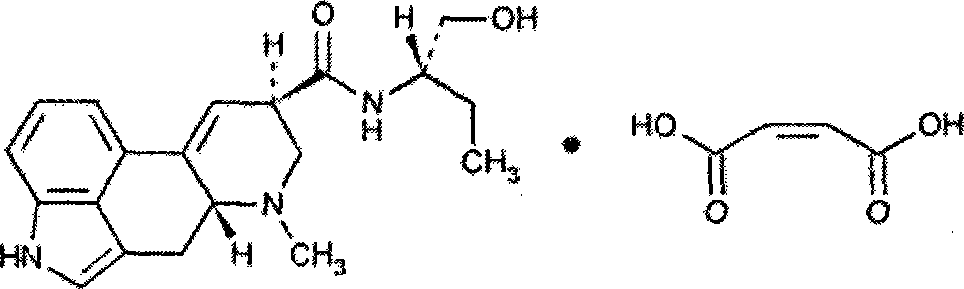
Methylergometrine Maleate powder injection and preparation method thereof
InactiveCN101125125AImprove complianceLess irritatingOrganic active ingredientsPowder deliveryErgometrineIrritation
The present invention relates to a methylergometrine maleate powder injection, which pertains to the field of pharmaceutical preparations; the present invention contains the methylergometrine maleate which is taken as the active ingredient and the pharmaceutically acceptable carriers, wherein, the weight ratio of the methylergometrine maleate and the pharmaceutically acceptable carriers is 1: 15 to 1: 300, the pharmaceutically acceptable carriers are one or more of the lactose, glucose, mannitol, low molecular weight dextran, sodium chloride and aminoacetic acid. The present invention also provides a preparation method of methylergometrine maleate powder injection. The powder injection of the present invention has good stability, easy storage and transportation, furthermore, the irritation is significantly decreased and the compliance of the patients is increased.
Owner:韩志强
Preparation method of iron-dextrin
ActiveCN107201387AUniform molecular weight distributionHigh quality raw materialFermentationSolubilityIron dextran
The invention discloses a preparation method of iron-dextrin. The preparation method comprises the following steps: preparing low molecular weight dextran through bi-enzyme (dextransucrase-dextranase) synergy, and then oxidizing dextran by taking sodium hypochlorite mixed liquor as an oxidizing agent, so as to obtain an oxidation-type dextran; simultaneously titrating and complexing with weak base and acid-base to prepare iron-dextrin and curing iron-dextrin under the conditions of high temperature and high pH value, so as to obtain cured iron-dextrin; filtering unreacted dextran and micromolecule iron-dextrin and ions therein with biological membranes with different molecular weights, so as to obtain purified iron-dextrin. The iron-dextrin prepared by the invention is uniform in distribution of the molecular weight, high in stability and less in content of impurities. Various indexes of iron-dextrin synthesized by the method conform to the requirements of Chinese pharmacopoeia, and the solubility of iron-dextrin is very good.
Owner:HEFEI UNIV OF TECH
One-step fermentation production process for low molecular weight dextran
InactiveCN102816812AControl synthesisReduce technical difficultyMicroorganism based processesFermentationBiotechnologyLeuconostoc mesenteroides
The present invention discloses a one-step fermentation production method for low molecular weight dextran. The production process comprises the following steps: inoculating Leuconostoc mesenteroides into a sucrose culture medium, and carrying out fermentation for 16-24 h to synthesize a fermentation liquid containing a dextran crude product; adding dextran enzyme to the fermentation liquid until a final dextran enzyme concentration is 0.05-5 U / mL, continuously carrying out fermentation, and carrying out the reaction for 2-8 h; after completing the reaction, carrying out inactivation to obtain a dextran crude product fermentation liquid; and carrying out a post-treatment on the crude product fermentation liquid to obtain the dextran finished product. Compared with traditional methods, the production process of the present invention has advantages of low technology difficulty, simple equipment, process simplification, and good control of low molecular weight dextran synthesis.
Owner:GUANGZHOU SUGARCANE IND RES INST +1
Low-molecular-weight glucan, its preparation method and use
InactiveCN101676304ASignificant immune enhancing activityOrganic active ingredientsImmunological disordersDrugChemistry
The invention discloses a low-molecular-weight glucan, characterized in that the glucan is obtained by separating from radix astragali. The invention also discloses a method for separating and purifying the low-molecular-weight glucan. In addition, the invention discloses a use of the low-molecular-weight glucan in the preparation of a medicament for the immunopotentiation and the adjutant therapyof tumor or a health food.
Owner:SHANGHAI INST OF PHARMA IND
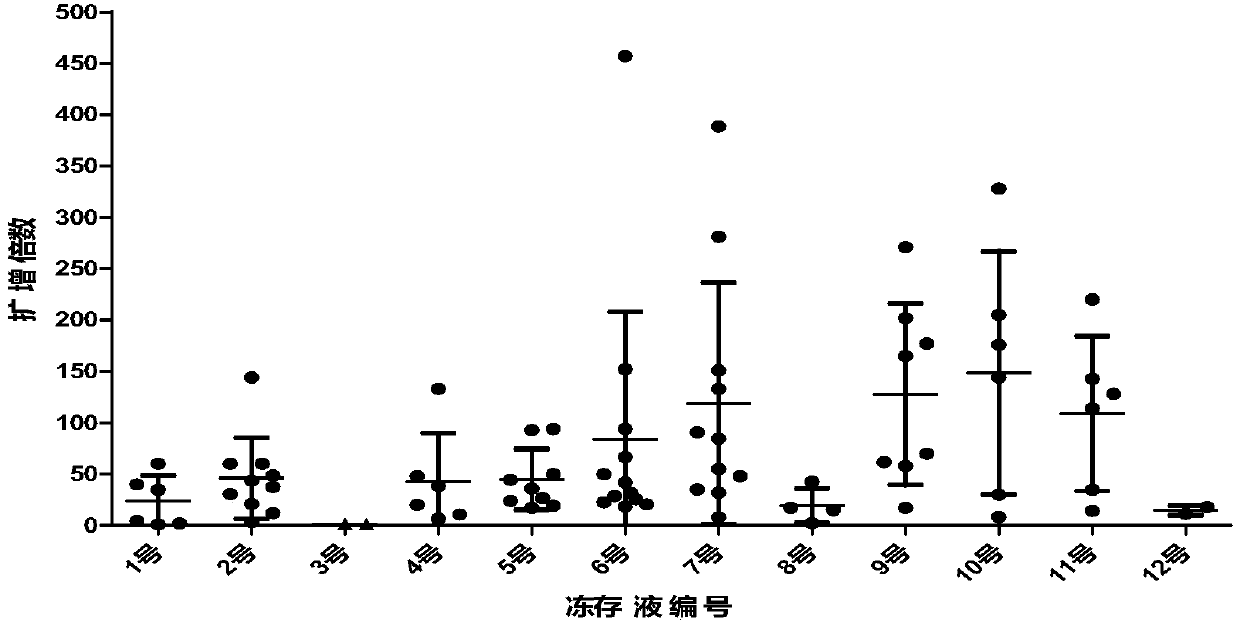
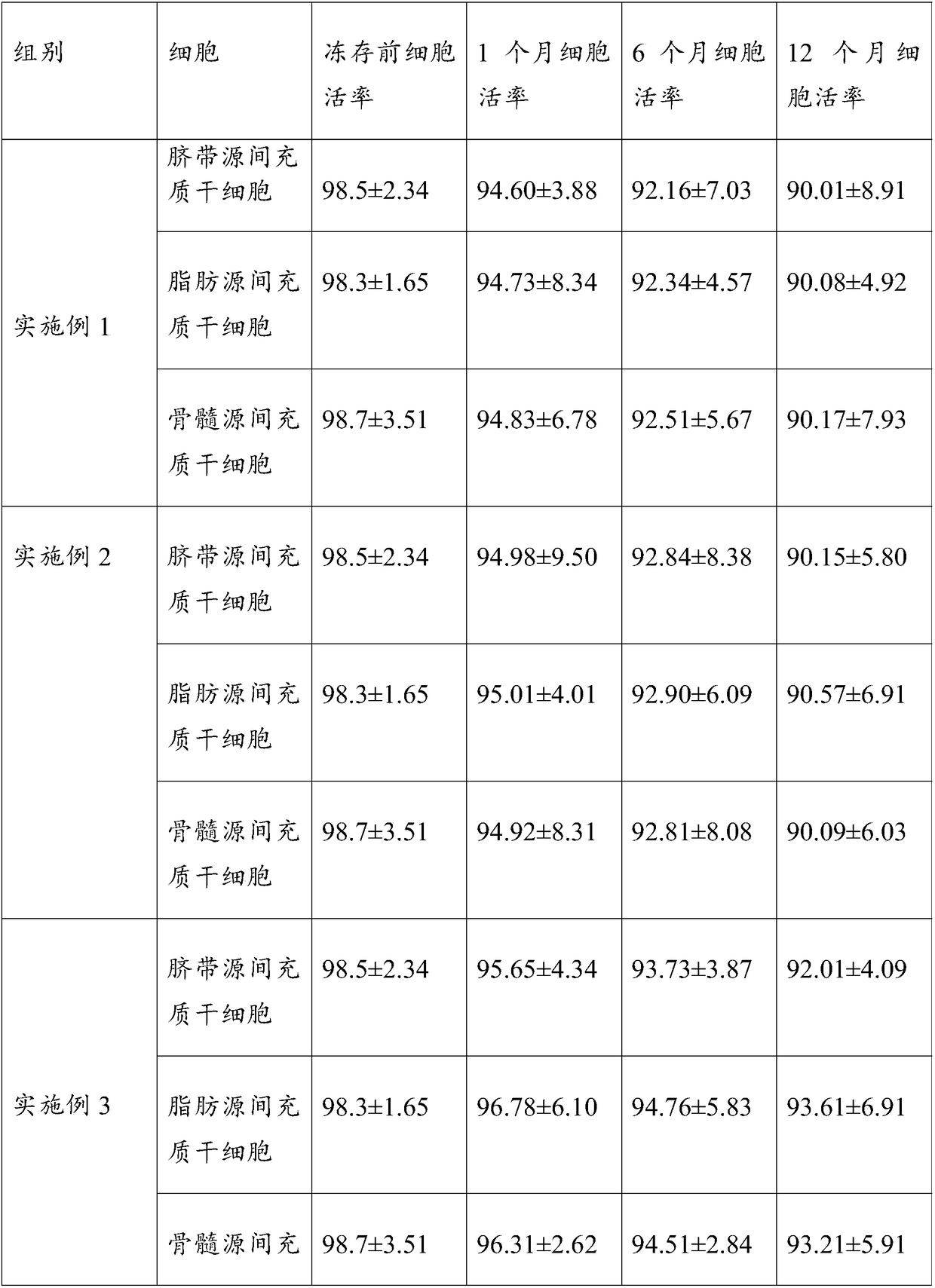
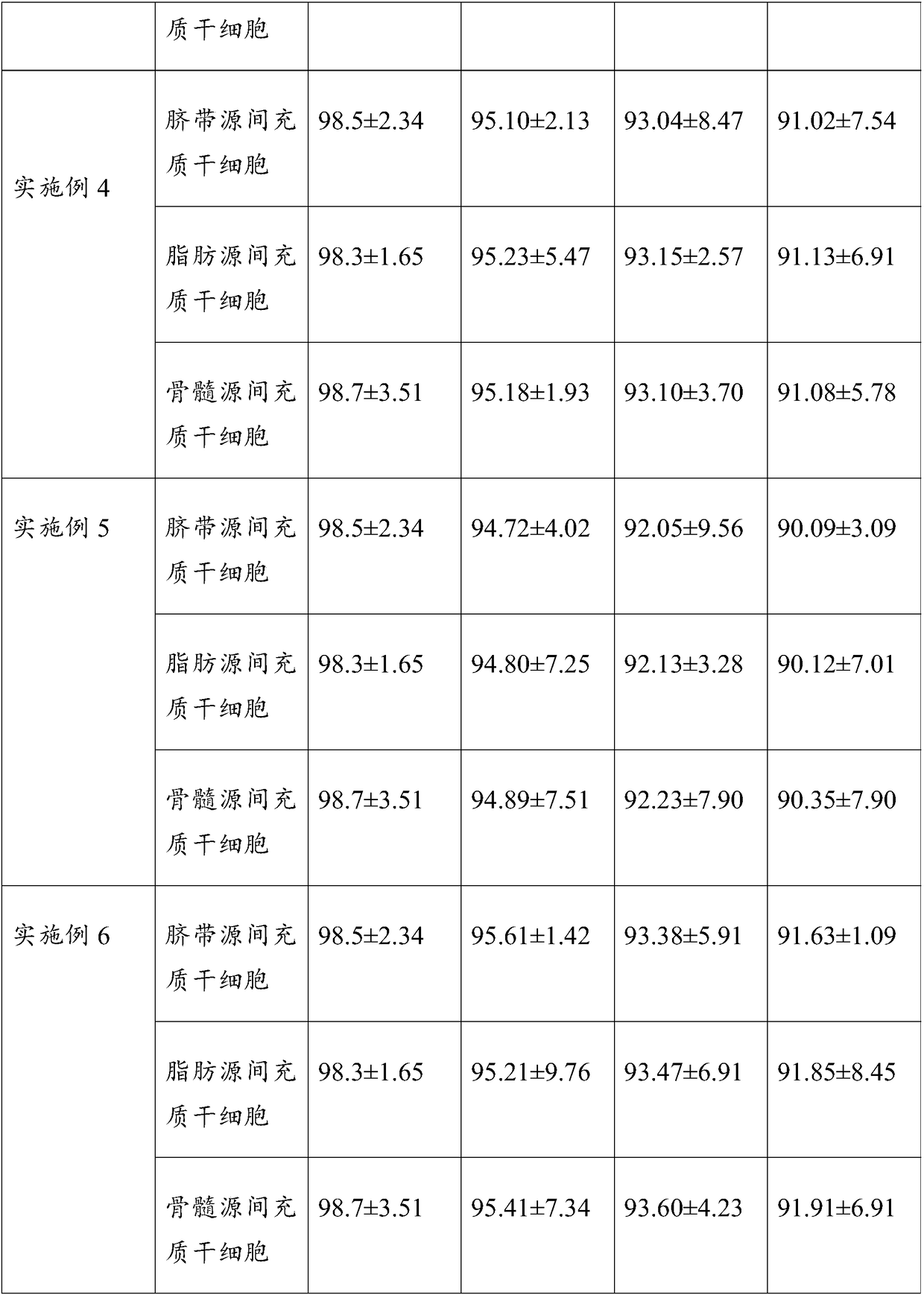
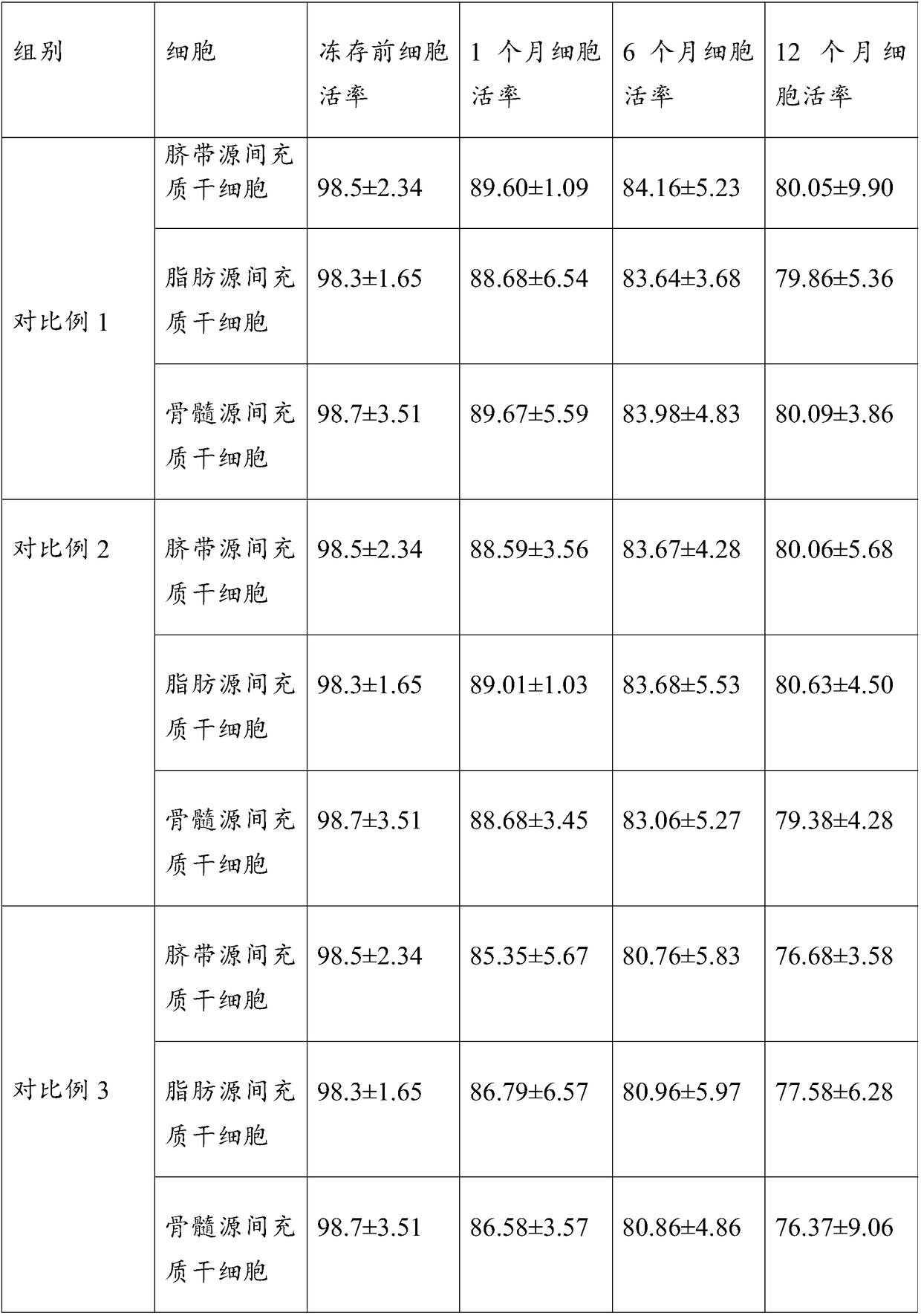
Cryopreserving liquid for cryopreserving single karyocyte
ActiveCN108029679AReduce the amount of introductionReduced Possibility of ContaminationDead animal preservationForeign proteinSerum ige
The invention discloses cryopreserving liquid for cryopreserving a single karyocyte. The cryopreserving liquid is prepared from fetal bovine serum, dimethyl sulfoxide and dextran dissolving liquid, wherein the fetal bovine serum accounts for 45 to 95 percent; the dextran dissolving liquid accounts for 10 to 50 percent; the dimethyl sulfoxide accounts for 5 to 10 percent. Dextran refers to low-molecular-weight dextran; a molecular weight of the dextran is 40,000. The cryopreserving liquid uses the low-molecular-weight dextran to replace part of animal serum, and can be used for effectively reducing an introduction amount of foreign protein. The cryopreserving liquid provided by the invention can be used for effectively retaining the cell activity of the single karyocyte which is directly cryopreserved after being extracted. If the single karyocyte is subjected to recovery culture at an interval after being subjected to cryopreservation treatment by using the cryopreserving liquid provided by the invention, a cell recovery rate can be maintained at a higher level, and further, the cell differentiation capacity is still high.
Owner:山东省齐鲁细胞治疗工程技术有限公司 +1
Cell freezing medium
InactiveCN108207934AOvercome limitationsEnhance immune functionDead animal preservationAntioxidantCulture mediums
The invention discloses a cell freezing medium. The cell freezing medium is prepared from the following components in percentage by volume and by concentration: 2 to 8 percent of dimethyl sulfoxide, 0.5 to 1.5 mg / ml of pawpaw extracting solution, 2.0 to 5mg / ml of chitosan quaternary ammonium salt, 0.5 to 2 percent of low molecular weight dextran, 8 to 15 percent of human serum albumin, 1.0 to 5mg / ml of glucan and the balance of DMEM culture medium. According to the cell freezing medium disclosed by the invention, the chitosan quaternary ammonium salt, the glucan and the pawpaw extracting solution are added, so that the limitation that chitosan can be only dissolved in a weak acid solution can be overcome; in addition, the effect of the cell freezing medium is better; by means of the glucan, the extracellular osmotic pressure can be improved and intracellular free water is reduced; due to the adoption of a permeable protective agent DMSO, cell rupture can be prevented, the melting pointof the intracellular water is reduced, and the formation of crystals is prevented; the permeable protective agent complements with a non-permeable protective agent glucan and the chitosan quaternaryammonium salt, so as to realize synergistic effects. The pawpaw extracting solution is a novel efficient natural antioxidant substance which is extracted from pawpaw and cannot be synthesized in a human body. The pawpaw extracting solution mainly plays an antioxidant role, and has the effects of improving cellular immune functions and prolonging cell activity.
Owner:广州瑞贝斯药业有限公司
Protection solution for preparing decellularized corneas
ActiveCN106212442ALow transparencyAvoid breedingDead animal preservationCell-Extracellular MatrixAdditive ingredient
The invention provides a protection solution for preparing decellularized corneas. The protection solution is a PBS or HBSS buffer solution, wherein 5-12 g / L of hyaluronic acid, 5-20 g / L of chondroitin sulfate, and 3-10 g / L of low molecular weight dextran are added in the PBS or HBSS buffer solution. According to the protection solution, protective ingredients are added in the basic buffer solution, the prepared protection solution can maintain the colloid osmotic pressure of a preparing solution of decellularized corneas through selection of components and a proportion, and compared with a traditional preparing solution for decellularized corneas, cornea transparency decreasing caused by excessive swelling and missing of extracellular matrix ingredients such as collagen in the decellularization treatment process in a later stage can be remarkably relieved; besides, antibiotic ingredients such as tobramycin are added, breeding of bacteria such as pseudomonas aeruginosa, klebsiella biofilm, enterobacter and proteus which are likely to infect corneas is suppressed, and the contamination probability in the treatment process is avoided.
Owner:SHENZHEN AINEAR CORNEA ENG
Preparation method and application of low molecular weight dextriferron
ActiveCN110183548AGood choiceImprove efficiencyHeavy metal active ingredientsBlood disorderGlucansucraseDEXTRIFERRON
The invention provides a preparation method and application of dextriferron with a weight average molecular weight between 1500 and 4000 Da. Specifically, maltose and sucrose are used as substrates, alow molecular weight dextran solution is prepared by complexing the maltose and sucrose with ferric trichloride under the action of dextransucrase, and a dextriferron solution is used to prepare a low molecular weight dextriferron injection. According to the preparation method of the invention, the conventional macromolecular degradation method is replaced with a small molecule polymerization manner to prepare the low molecular weight dextriferron injection, the cheap maltose and sucrose are used as the substrates, so that the preparation cost is low; moreover, by adjusting the ratio of the substrates, i.e., maltose and sucrose, dextriferron with different sugar chain lengths is prepared; a complex catalyst is introduced into the complexation process of iron to improve the complexation efficiency; the obtained low molecular weight dextran injection has the advantages of small irritancy, good absorption and iron supplementation effect and the like, and can be used as a preferred iron supplement agent for pigs, cattle, sheep and other livestock.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Low-molecular-weight glucan, its preparation method and use
InactiveCN101676303ASignificant immune enhancing activityOrganic active ingredientsImmunological disordersGlucanImmunopotentiation
The invention discloses a low-molecular-weight glucan, characterized in that the glucan is obtained by separating from radix astragali. The invention also discloses a method for separating and purifying the low-molecular-weight glucan. In addition, the invention discloses a use of the low-molecular-weight glucan in the preparation of a medicament for the immunopotentiation and the adjutant therapyof tumor or a health food.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
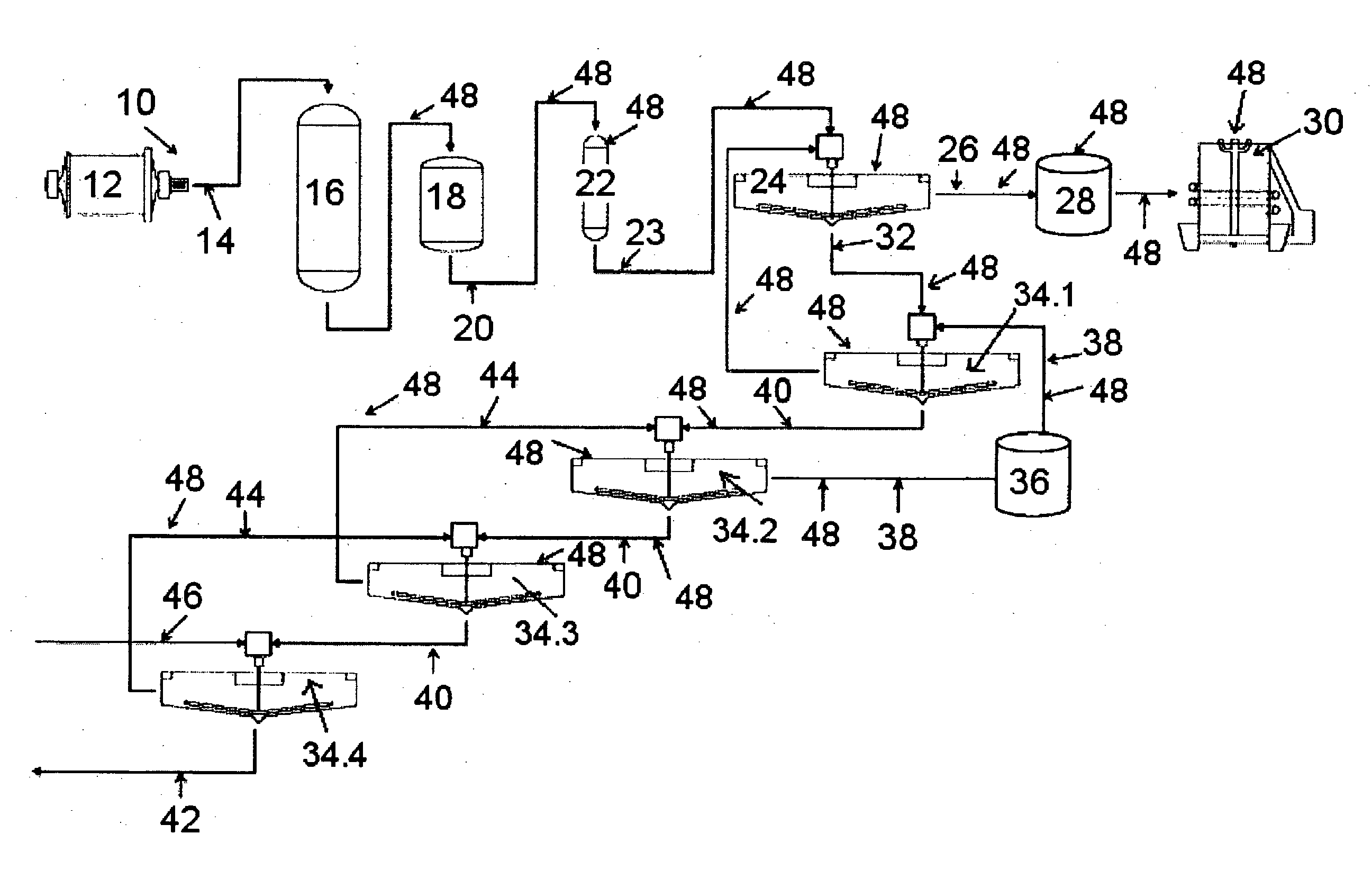
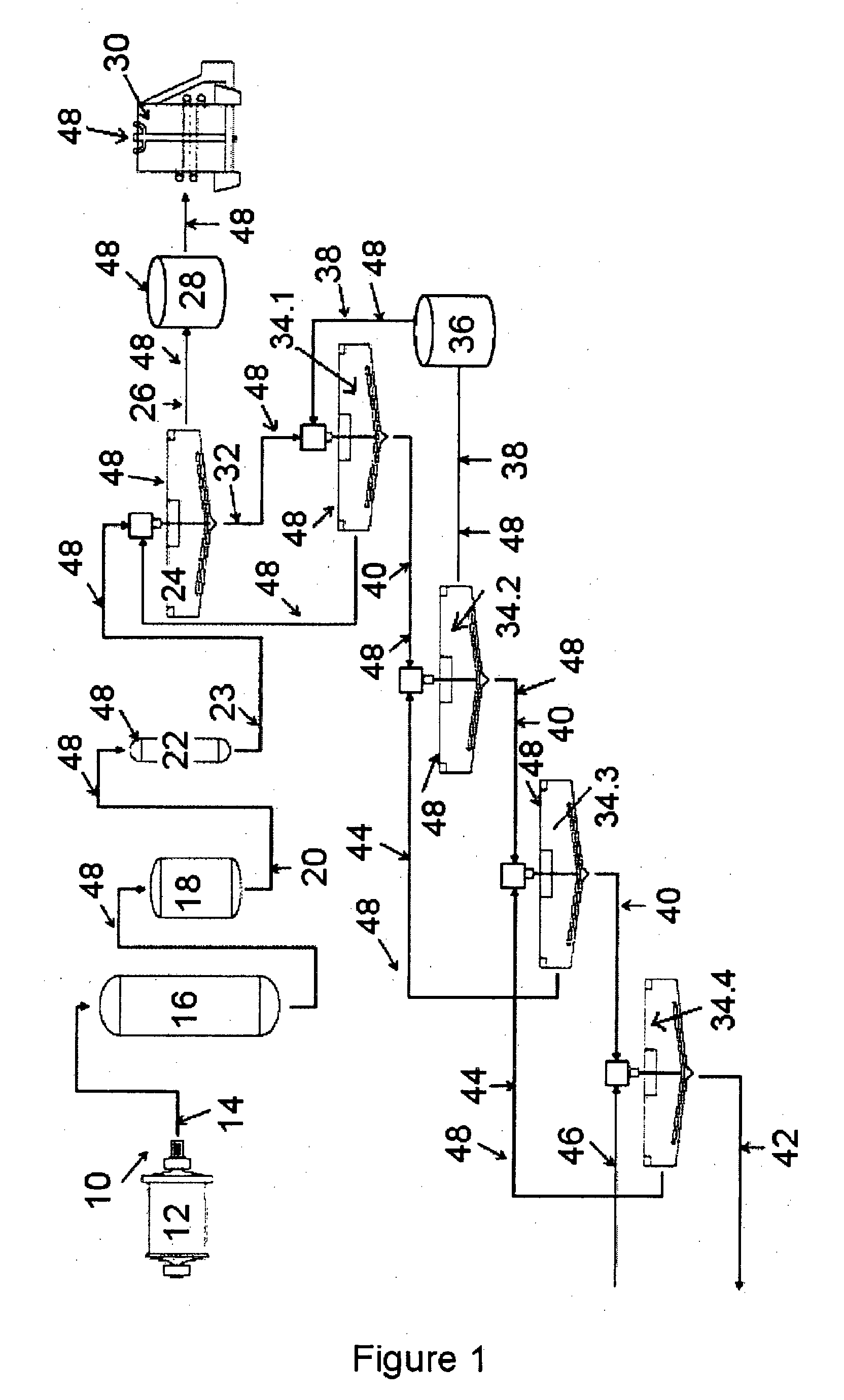
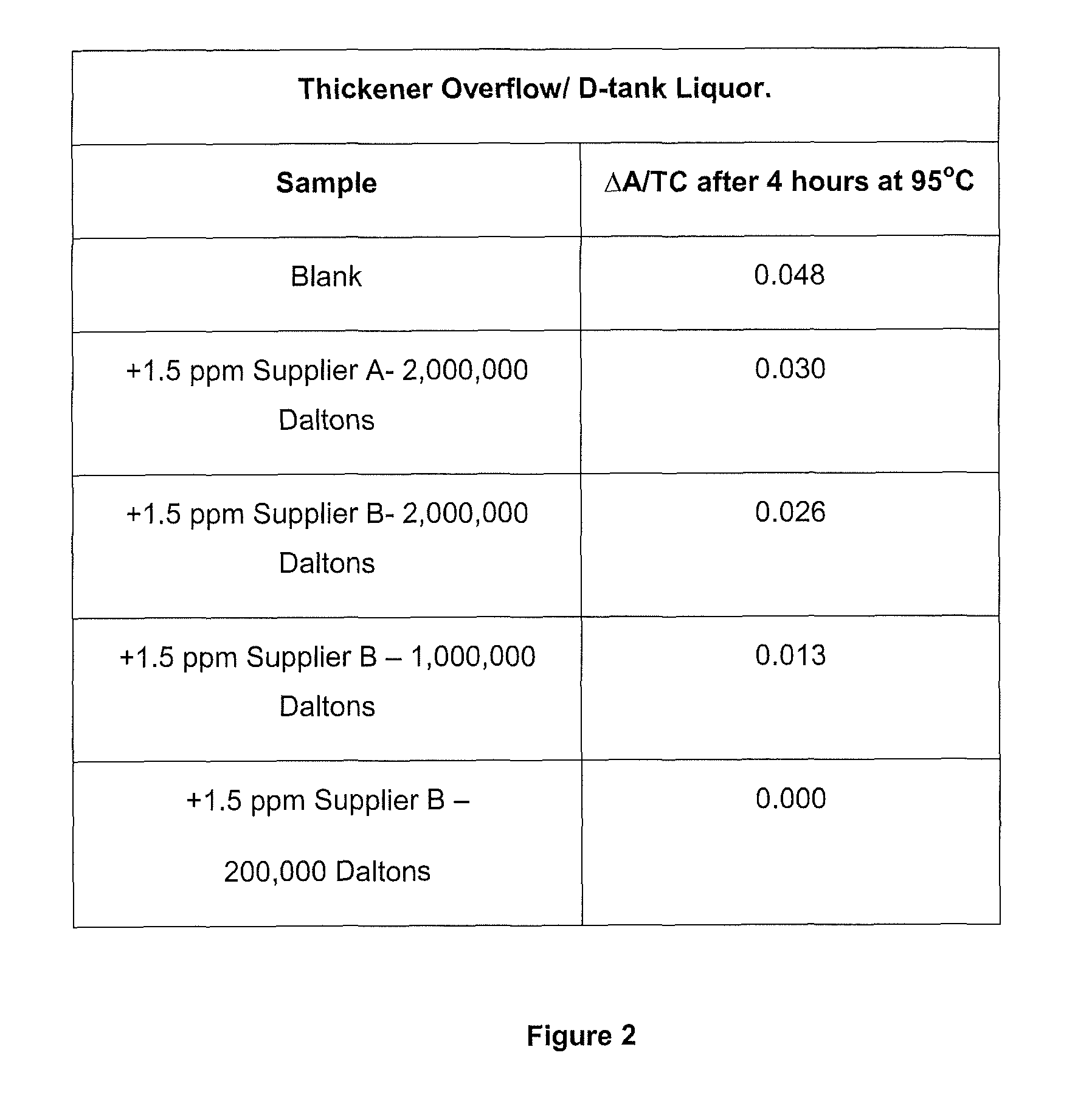
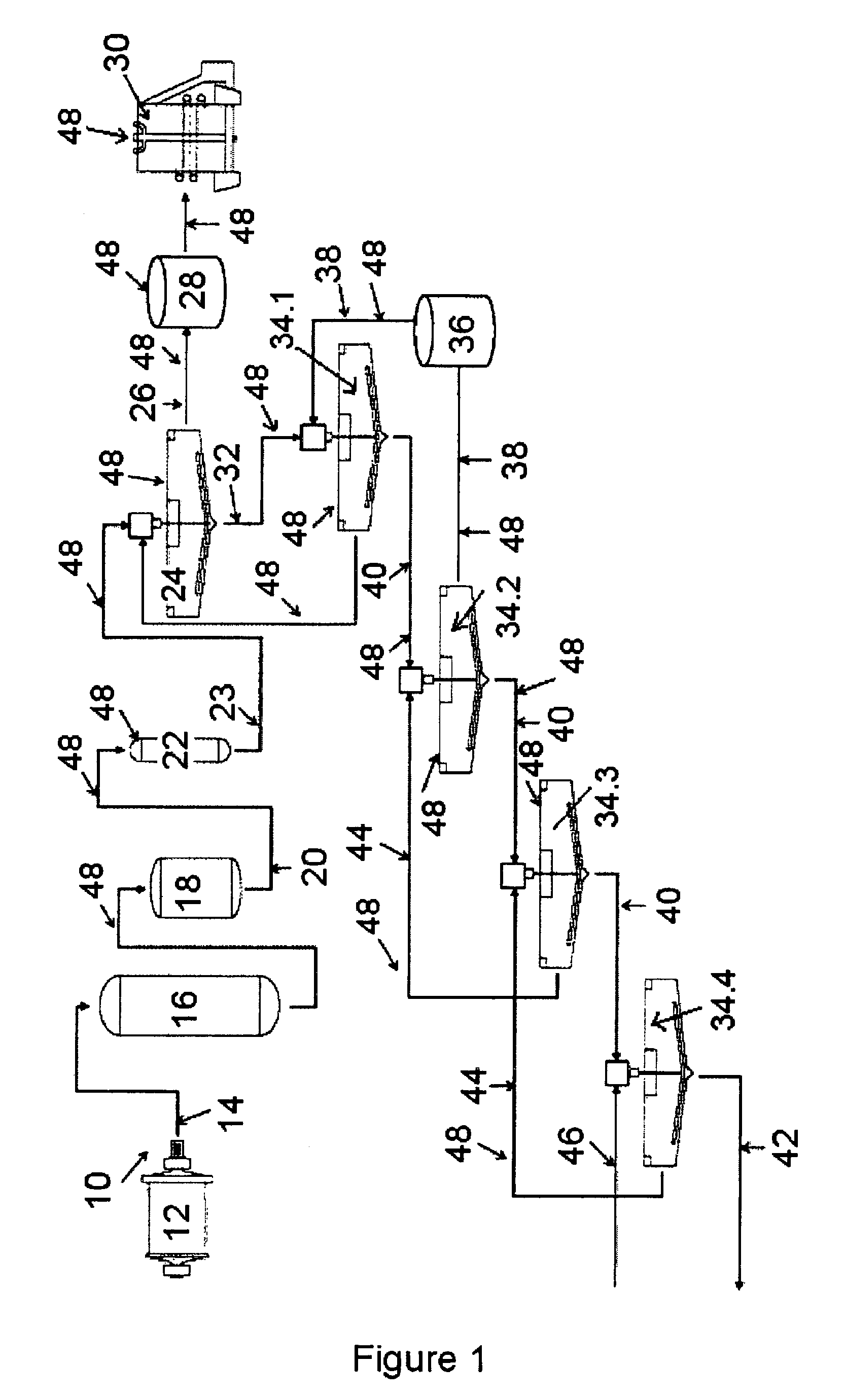
Method of increasing the stability of a bayer process liquor
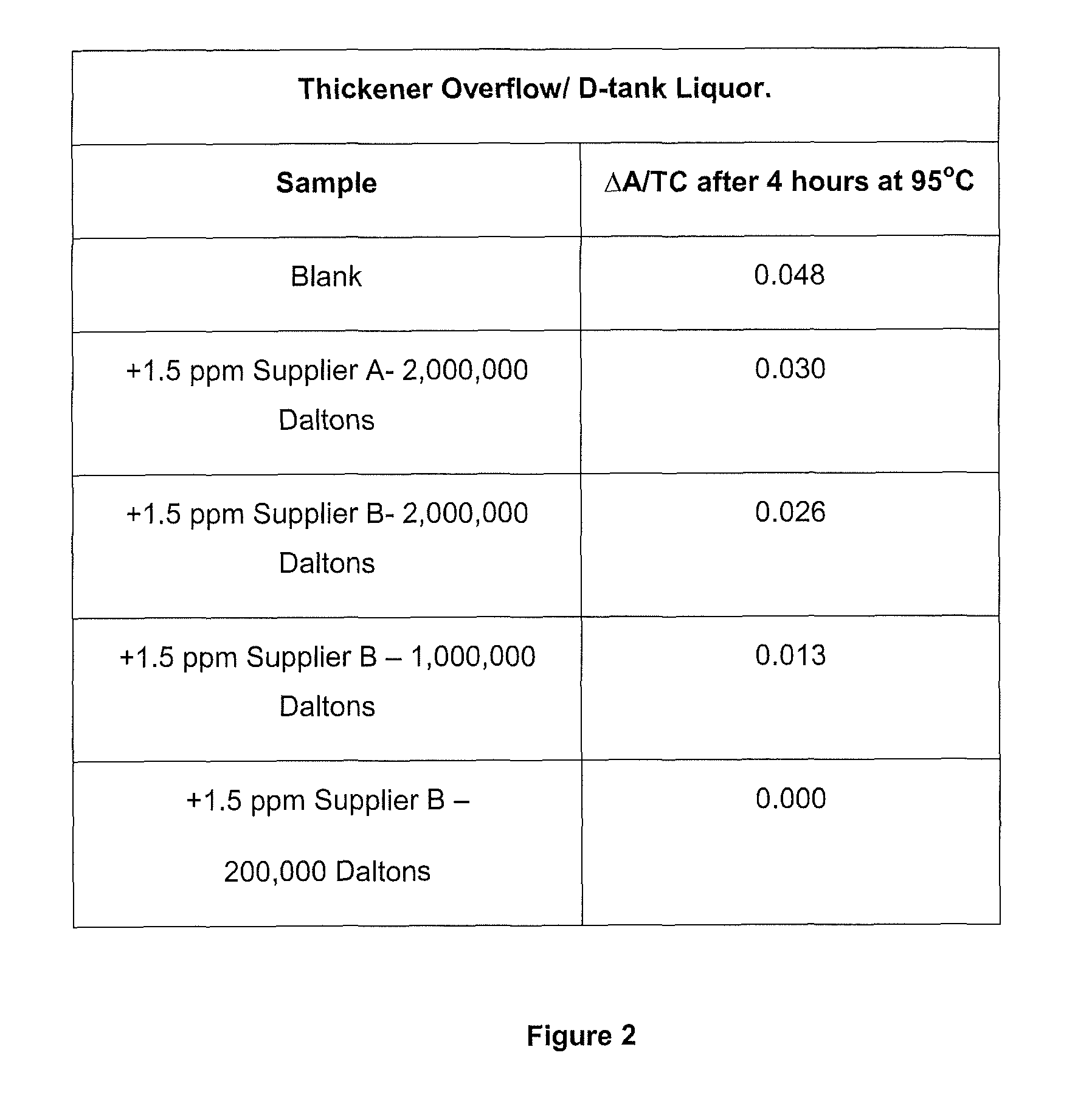
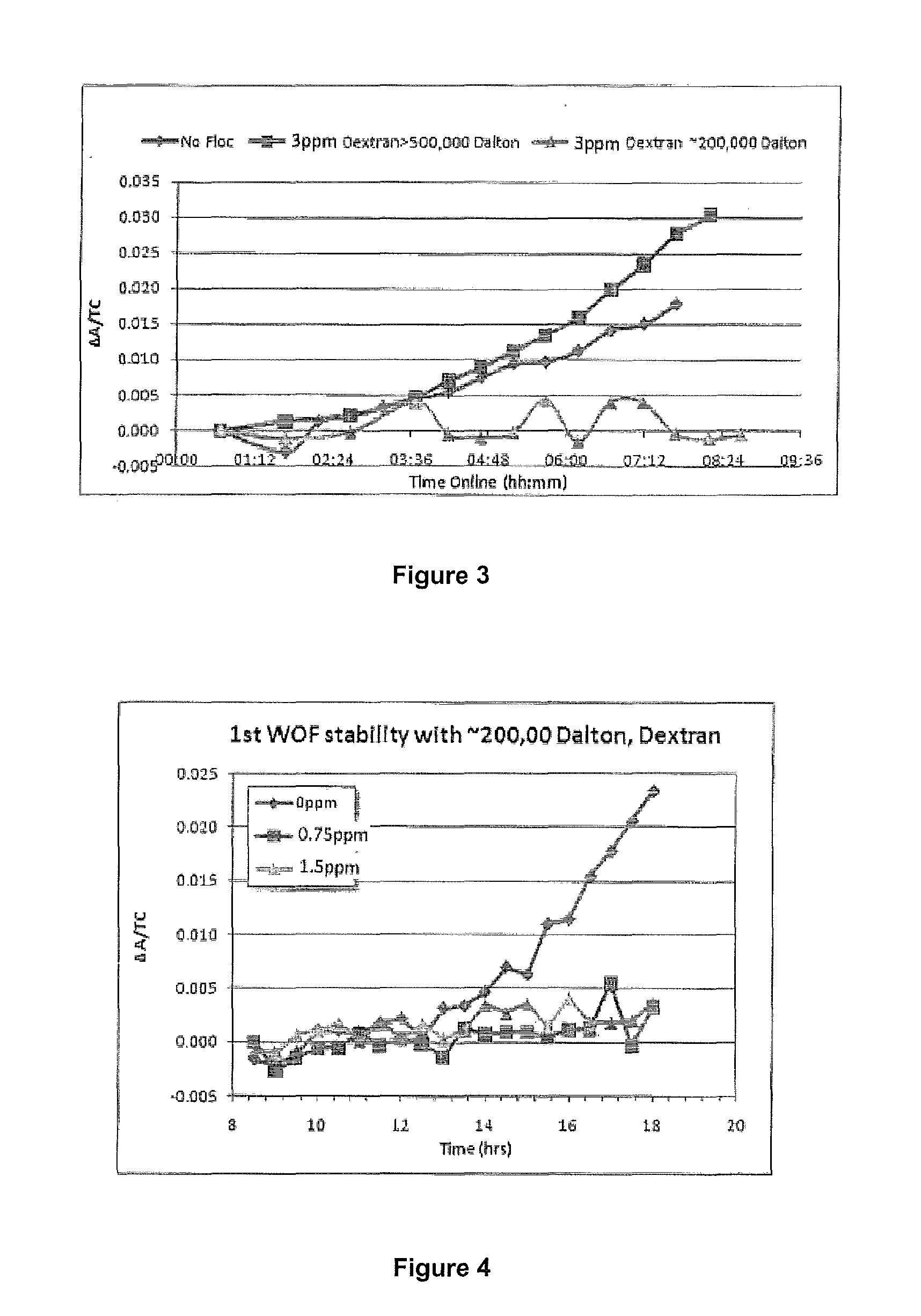
ActiveUS20140010742A1Increase liquor stabilityReduce molecular weightGallium/indium/thallium compoundsAluminates/aluminium-oxide/aluminium-hydroxide purificationChemistryLow molecular weight dextran
Methods, and related products and compositions, of increasing the stability of a Bayer process liquor are described. A method of increasing the stability of a Bayer process liquor includes contacting the Bayer process liquor with a ppm quantity of a low molecular weight dextran. Also described are methods, and related products and compositions, for controlling the precipitation of aluminium-containing compounds from a Bayer process liquor.
Owner:ALCOA OF AUSTRALIA LTD
Method of increasing the stability of a Bayer process liquor
ActiveUS9187337B2Improve stabilitySuppresses precipitationAluminates/aluminium-oxide/aluminium-hydroxide purificationAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationChemistryLow molecular weight dextran
Methods, and related products and compositions, of increasing the stability of a Bayer process liquor are described. A method of increasing the stability of a Bayer process liquor includes contacting the Bayer process liquor with a ppm quantity of a low molecular weight dextran. Also described are methods, and related products and compositions, for controlling the precipitation of aluminum-containing compounds from a Bayer process liquor.
Owner:ALCOA OF AUSTRALIA LTD
Umbilical cord blood hematopoietic stem cell preservation solution and preparation method thereof
ActiveCN110839612BLow toxicityAvoid problems such as immune allergiesDead animal preservationCord blood stem cellGlycerol
The invention provides a cord blood hematopoietic stem cell preservation solution and a preparation method thereof, belonging to the technical field of cell preservation. The raw materials of the preservation solution include: low molecular weight dextran, dimethyl sulfoxide, DMEM medium, glycerol, folic acid, mannose Polysaccharide and phosphatidylserine are mixed with raw materials to obtain a preservation solution, and then mixed with hematopoietic stem cells after two stages of variable temperature pretreatment to preserve hematopoietic stem cells. The prepared preservation solution has a long preservation time, the preserved stem cells have less cell damage after resuscitation, and the survival rate of the stem cells is high, and the preparation and preservation methods are simple and inexpensive.
Owner:HUNAN YUANPIN CELL TECH CO LTD
Method for preparing medium-low molecular weight dextran by using biological method
ActiveCN102690857BHigh yieldReduce usageCosmetic preparationsBiocideMicroorganismLeuconostoc mesenteroides
Owner:GUANGXI UNIV FOR NATITIES
Method for preparing cross-linked dextran gels resistant to alpha-glucosidase hydrolysis
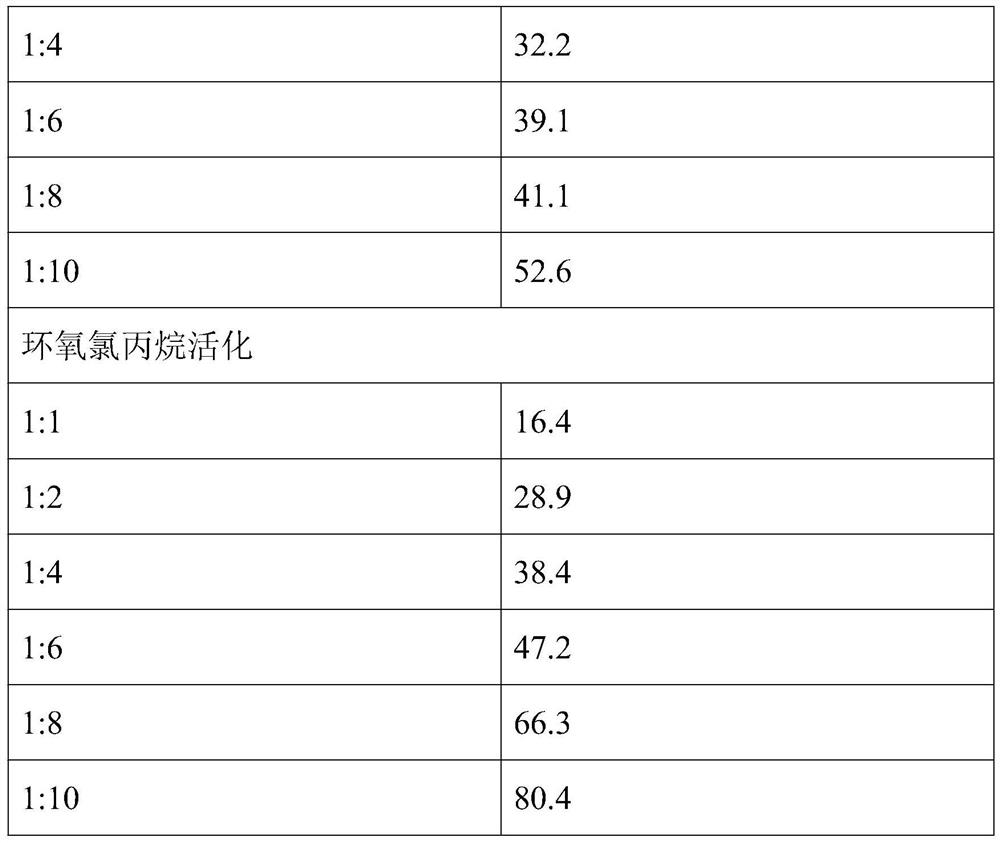
The present invention provides a method for preparing a cross-linked dextran gel resistant to hydrolysis by α-glucosidase. The low-molecular-weight dextran is used as the starting skeleton, and an activator containing an epoxy group is used to activate it to make It has a plurality of epoxy groups, activated low-molecular-weight dextran; the epoxy-containing activator is 1,4-butanediol diglycidyl ether, ethylene glycol diglycidyl ether or epoxy A kind of chloropropane; using activated low molecular weight dextran as a biological cross-linking intermediate to mix with high molecular weight dextran and carry out a cross-linking reaction to form a hydrogel, and homogenize to form uniform gel particles; adding N -Acetyl-D-glucosamine, D-glucosamine, and cellobiose functional monomers. In the gel prepared by the method, the catalytic efficiency of α-glucosidase is reduced by more than 50% in the experiment of in vitro dextran hydrolysis.
Owner:宁夏妙朗生物科技有限公司
Biological film type preserving fluid and preparation method thereof
InactiveCN109997840AProtect biological functionSave clearDead animal preservationVitamin CBULK ACTIVE INGREDIENT
The invention provides a biological film type preserving fluid. Every 1 L of the preserving fluid is prepared from the following components: 300-700 ml of an MEM cell culture liquid, 0-5 g of low molecular weight dextran, 0-2 g of hyaluronic acid with the molecular weight of 5-300 w and a double-antibody containing 20-200 mg / L of penicillin and streptomycin. In addition, every 1 L of the preserving fluid contains 300-700l ml of glycerine and / or 1,3-propylene glycol and or one or more of 0-10 g / L cysteine, 0-10 g / L vitamin C, 0-1g / L procyanidine and 0-3 g / L glutathione. The preserving fluid hasthree functions of protecting active ingredients in a biological film in an irradiation sterilization deactivating process, protecting the structure of the biological film in freezing and preservingperiods and protecting the biological function of the biological film in the preserving period. The invention also provides a preparation method of the biological film type preserving fluid which is convenient to prepare, good in economical benefit and clear in function, covers a production process and a preserving stage of the biological film and is suitable for long-term preservation of a wet biological film in a frozen state.
Owner:广州锐澄医疗技术有限公司
A kind of protective solution for acellular cornea preparation
ActiveCN106212442BLow transparencyAvoid breedingDead animal preservationCell-Extracellular MatrixAdditive ingredient
The invention provides a protection solution for preparing decellularized corneas. The protection solution is a PBS or HBSS buffer solution, wherein 5-12 g / L of hyaluronic acid, 5-20 g / L of chondroitin sulfate, and 3-10 g / L of low molecular weight dextran are added in the PBS or HBSS buffer solution. According to the protection solution, protective ingredients are added in the basic buffer solution, the prepared protection solution can maintain the colloid osmotic pressure of a preparing solution of decellularized corneas through selection of components and a proportion, and compared with a traditional preparing solution for decellularized corneas, cornea transparency decreasing caused by excessive swelling and missing of extracellular matrix ingredients such as collagen in the decellularization treatment process in a later stage can be remarkably relieved; besides, antibiotic ingredients such as tobramycin are added, breeding of bacteria such as pseudomonas aeruginosa, klebsiella biofilm, enterobacter and proteus which are likely to infect corneas is suppressed, and the contamination probability in the treatment process is avoided.
Owner:SHENZHEN AINEAR CORNEA ENG
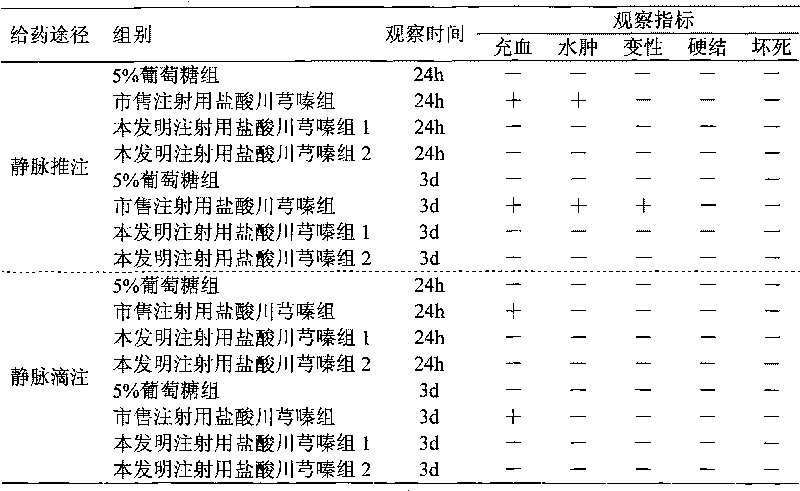
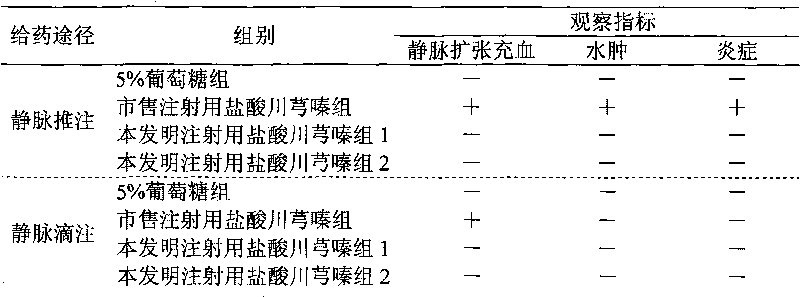
Drug composition of ligustrazine hydrochloride and benzyl alcohol and preparation method thereof
InactiveCN101756989ALess irritatingNon-irritatingPowder deliveryOrganic active ingredientsVascular diseaseMANNITOL/SORBITOL
The invention belongs to the technical field of medicine, and discloses a drug composition of ligustrazine hydrochloride and benzyl alcohol and a preparation method thereof. The weight ratio of the ligustrazine hydrochloride and the benzyl alcohol is 1:0.01 to 0.1, and an injection is prepared by the drug composition and pharmaceutically acceptable excipients; and the excipients are selected from one or more of mannitol, sorbitol, low-molecular-weight dextran or sodium chloride. The drug composition can be used for treating cerebral vascular diseases, overcomes the defects that the ligustrazine hydrochloride injection has greater irritation in the process of clinical administration in the prior art, and is favorable for expanding clinical medication.
Owner:HAINAN SIHUAN PHARMA +1
Additive for tablets
InactiveUS20070071810A1Bondability be controlledTimely controlBiocideOrganic active ingredientsDispersityPolymer science
A disintegrant for tablets consisting of an α-1,4-glucan having a degree of polymerization of not less than 180 and less than 1230 and a dispersity (weight average molecular weight “Mw” / number average molecular weight “Mn”) of not more than 1.25 or a modified product thereof. A binder for tablets consisting of an α-1,4-glucan having a degree of polymerization of not less than 1230 and not more than 37000 and a dispersity of not more than 1.25, or a modified product thereof. A binding-disintegrating agent for tablets consisting of a low molecular weight α-1,4-glucan or a modified product thereof, and a high molecular weight α-1,4-glucan or a modified product thereof, wherein the low molecular weight α-1,4-glucan has a degree of polymerization of not less than 180 and less than 1230 and a dispersity of not more than 1.25, and wherein the high molecular weight α-1,4-glucan has a degree of polymerization of not less than 1230 and not less than 37000 and a dispersity of not more than 1.25.
Owner:EZAKI GLICO CO LTD +1
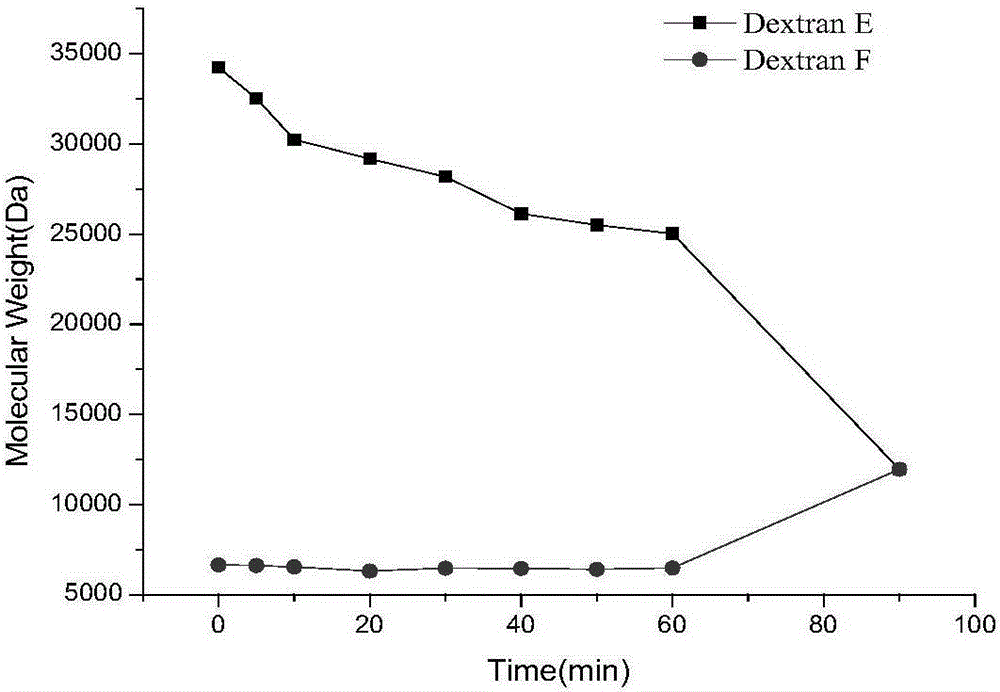
A kind of dextranase and its application in preparing low molecular dextran
ActiveCN103834574BReduce energy consumptionHigh yieldFungiMicroorganism based processesBiotechnologyMicroorganism
The invention discloses a preparation method for dextranase and application of the dextranase in preparation of low molecular dextran. The invention is characterized in that a producing bacterial strain for the dextranase is Penicillium aculeatum F1008 separated from soil and the bacterial strain is preserved in China General Microbiological Culture Collection Center with an accession number of CGMCC No. 8135. According to the invention, the dextranase with high enzyme activity is successfully prepared through fermentation culture of Penicillium aculeatum F1008; the dextranase has catalysis temperature of 25 to 35 DEG C, a catalysis pH value of 5.0 to 6.0 and average enzyme activity of higher than 600 U / mL, gives preference to reaction with a high-molecular-weight substrate in a multi-substrate solution and is especially applicable to preparation of low-molecular-weight dextran; low molecular dextran varieties prepared through catalysis of the Penicillium aculeatum F1008 dextranase comprise micromolecular dextran with weight average molecular weight of 10000 Da, 6000 to 8000 Da and 3000 to 5000 Da. The dextranase has the advantages of mild catalytic reaction condition, good substrate specificity, low energy consumption and capacity of achieving the objects consisting of yield increase, low carbon and low cost in production of micromolecular dextran.
Owner:HEFEI UNIV OF TECH
Preparation method of adenosine triphosphate coenzyme insulin freeze-dried powder injection
ActiveCN102038939BFull appearanceGood resolubilityOrganic active ingredientsPowder deliveryAdenosineArginine
The invention discloses a preparation method of an adenosine triphosphate coenzyme insulin freeze-dried powder injection, comprising the following steps of: adjusting injection water with diluted hydrochloric acid until the pH value is 2.5-3.5, adding insulin and uniformly mixing to obtain a solution I; adding L-arginine and disodium ethylene diamine tetraacetate in the injection water, uniformly stirring, adding carbon and stirring; after removing carbon, adding adenosine disodium triphosphate and uniformly stirring to obtain a solution II; adding calcium gluconate, low molecular weight dextran and cysteine hydrochloride in the injection water, adding carbon and removing the carbon and uniformly stirring to obtain a solution III; mixing three solutions, uniformly stirring, adjusting the pH value to be 5.7-6.2 with a 2-4 percent NaOH solution, adding coenzyme A and supplementing the balance of injection water; and sterilizing, measuring the content, subpackaging and freezing dry. The adenosine triphosphate coenzyme insulin freeze-dried powder injection prepared by the method has the advantages of favorable redissolution, clearness and quality stability of products and is saturated in appearance.
Owner:NORTH CHINA PHARMA COMPANY
A kind of preparation method of iron dextran
ActiveCN107201387BUniform molecular weight distributionThe process is simple and easy to controlFermentationIron dextranSynergy
The invention discloses a preparation method of iron-dextrin. The preparation method comprises the following steps: preparing low molecular weight dextran through bi-enzyme (dextransucrase-dextranase) synergy, and then oxidizing dextran by taking sodium hypochlorite mixed liquor as an oxidizing agent, so as to obtain an oxidation-type dextran; simultaneously titrating and complexing with weak base and acid-base to prepare iron-dextrin and curing iron-dextrin under the conditions of high temperature and high pH value, so as to obtain cured iron-dextrin; filtering unreacted dextran and micromolecule iron-dextrin and ions therein with biological membranes with different molecular weights, so as to obtain purified iron-dextrin. The iron-dextrin prepared by the invention is uniform in distribution of the molecular weight, high in stability and less in content of impurities. Various indexes of iron-dextrin synthesized by the method conform to the requirements of Chinese pharmacopoeia, and the solubility of iron-dextrin is very good.
Owner:HEFEI UNIV OF TECH
Clinical grade cell protection solution and its preparation method and application
ActiveCN106212443BDamage reliefImprove survival rateDead animal preservationClinical gradeCell survival
The invention relates to clinical-grade cellular protection liquid as well as a preparation method and application thereof. The clinical-grade cellular protection liquid contains a human serum albumin injection, a low-molecular-weight dextran and amino acid injection and an isotonic sodium chloride injection. The preparation method comprises the step of uniformly mixing the human serum albumin injection, the low-molecular-weight dextran and amino acid injection and the isotonic sodium chloride injection. Compared with the prior art, the preparation method has the advantages that a proper amount of the human serum albumin injection and a proper amount of the dextran and amino acid injection are creatively added into the isotonic sodium chloride injection, so that nutrients can be provided for cells, meanwhile, acid-base imbalance caused by cellular metabolism can be regulated, cellular damage caused due to acidosis can be relieved, the cell survival rate can be greatly increased, the cell viability can be maintained, the cell survival time is relatively long, the cells can be directly applied to clinical application, and the application is relatively safe and convenient; and the preparation method is simple, convenient and rapid.
Owner:四川驰鼎盛通生物科技有限公司
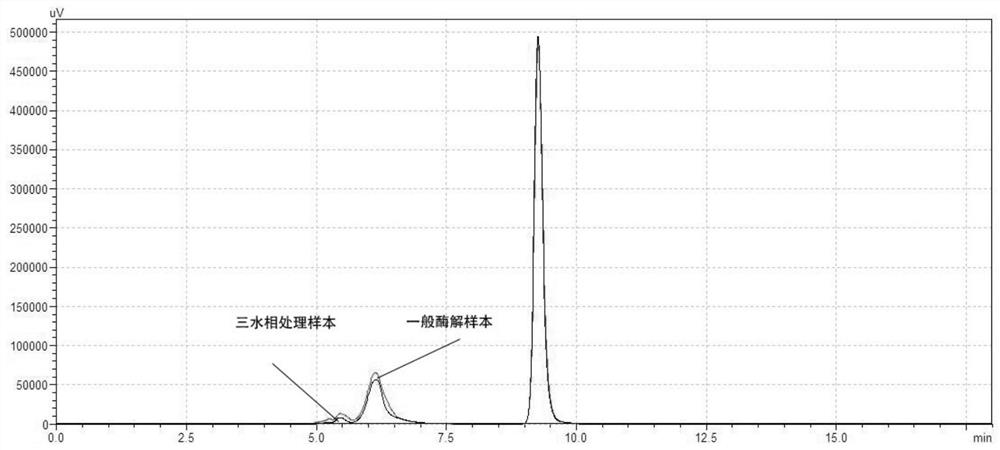
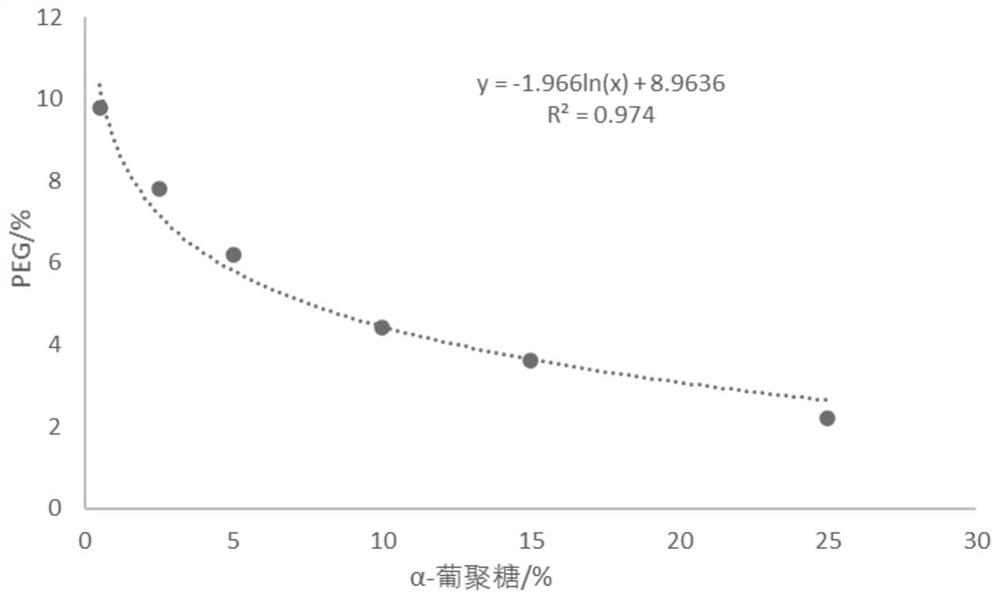
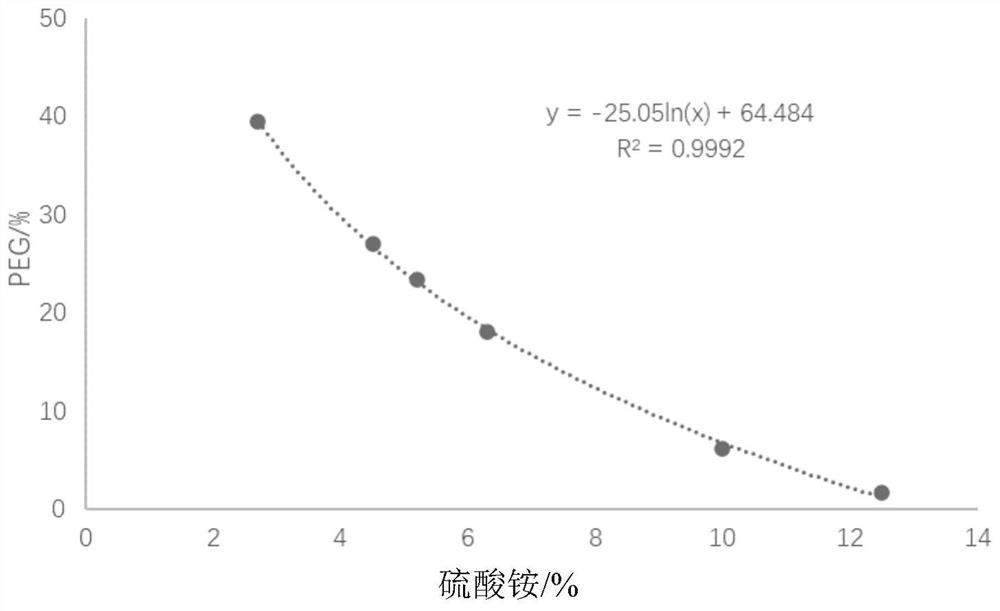
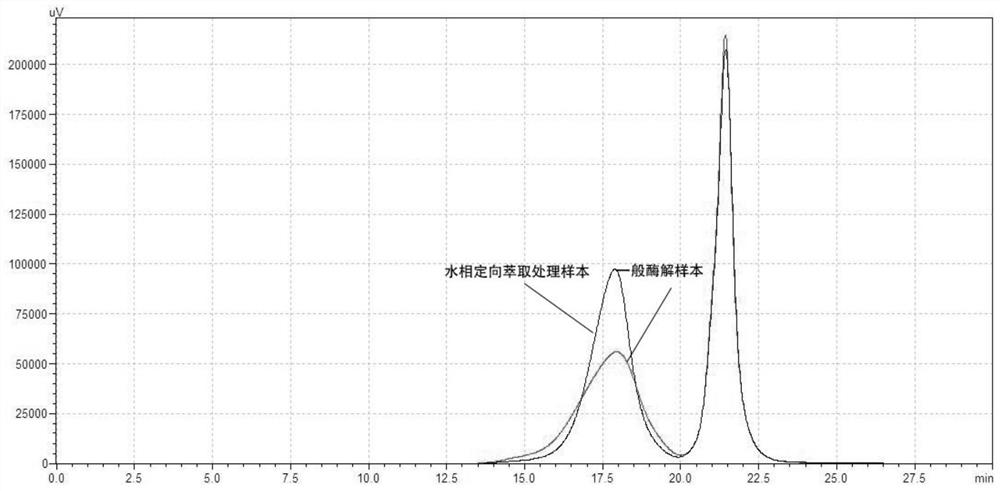
Method for generating alpha-glucan with specific molecular weight by utilizing three-aqueous-phase system to spontaneously regulate and control hydrolysis and application of alpha-glucan
The invention discloses a method for generating alpha-glucan with specific molecular weight through spontaneous regulation and hydrolysis of a three-aqueous-phase system and application of the method, and belongs to the technical field of enzymatic reaction regulation. Glucan is not only a polymer capable of forming a phase body, but also the molecular weight of glucan is a key factor influencing a phase forming condition, and the final target molecular weight of the enzymolysis reaction falls on a phase splitting point by adjusting the phase body composition of other high polymers, so that the purpose of spontaneously stopping the reaction is achieved. Meanwhile, the high partition coefficient of the alpha-glucanase in part of the polymer phase is utilized to quickly recover the zymoprotein from the reaction phase and stop the enzymolysis reaction, and after the low-molecular-weight alpha-glucan is separated, the zymoprotein is recovered again through a concentrated salt solution.
Owner:GUANGDONG ACAD OF SCI INST OF BIOENGINEERING
Method for accurately regulating and controlling alpha-glucan enzymolysis reaction and directionally extracting product with specific molecular weight and application of method
PendingCN114686543AEasy to recycleEasy to controlSugar derivativesFermentationGlucanaseEnzyme protein
The invention discloses a method for accurately regulating and controlling alpha-glucan enzymolysis reaction and directionally extracting a specific molecular weight product and application of the method, and belongs to the technical field of biochemical separation. Glucan is not only a polymer capable of forming a phase body, but also the molecular weight of glucan is a key factor influencing a phase forming condition, and the final target molecular weight of the enzymolysis reaction falls on a phase splitting point by adjusting the phase body composition of other high polymers. And meanwhile, the high partition coefficient of the alpha-glucanase in part of the polymer phase is utilized to quickly recover enzyme protein from the reaction phase and stop the enzymolysis reaction, and after the low-molecular-weight alpha-glucan is separated, the enzyme is recovered again through a specific concentrated salt solution which is compatible with the alpha-glucanase protein.
Owner:GUANGDONG ACAD OF SCI INST OF BIOENGINEERING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com