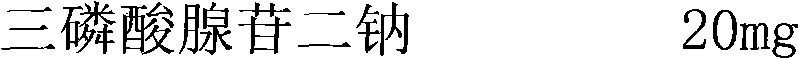Preparation method of adenosine triphosphate coenzyme insulin freeze-dried powder injection
A technology of adenosine triphosphate coenzyme and insulin, which is applied in the direction of freeze-drying transportation, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of poor product stability, long freeze-drying time, and collapsed appearance, and achieve Good clarity, stable product quality and full appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
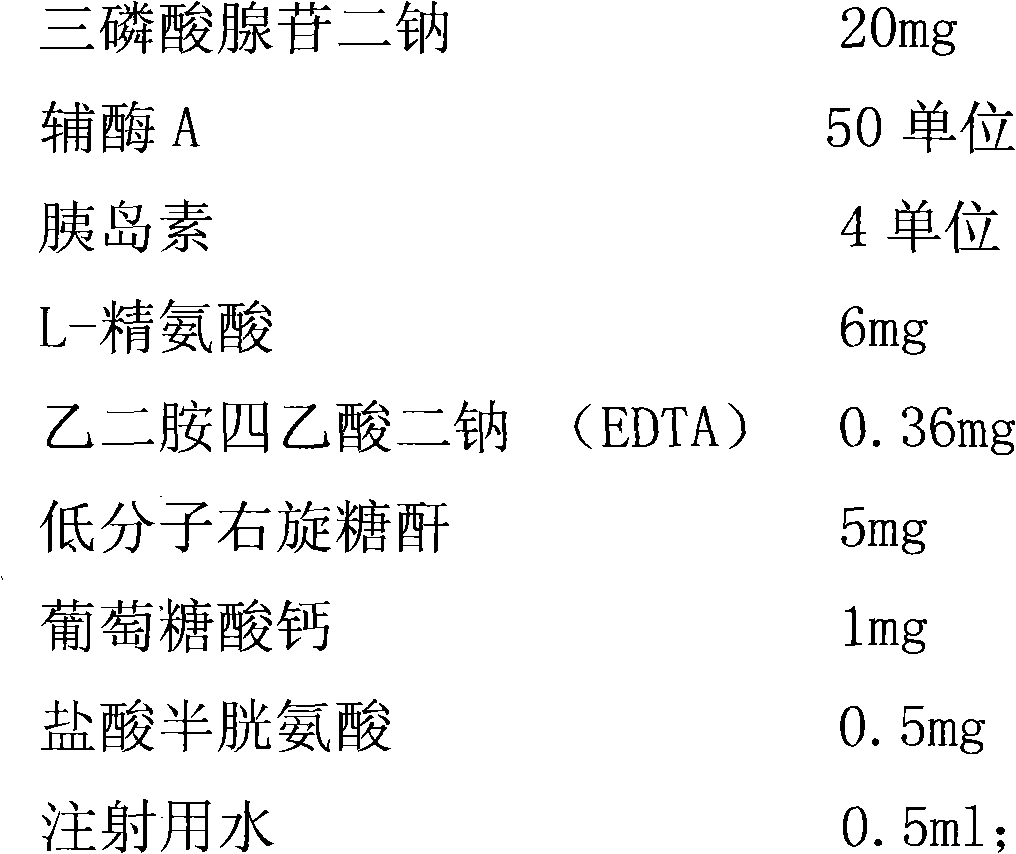
[0023] Weigh the main ingredient and auxiliary materials according to the following process formula:
[0024]
[0025] The specific preparation process is:
[0026] (a) Add an appropriate amount of water for injection into the stainless steel barrel, adjust the pH value to about 2.5 with 10% hydrochloric acid, add insulin, stir evenly, and prepare solution I for later use.
[0027] (b) Add an appropriate amount of water for injection into a stainless steel bucket, add L-arginine and EDTA, stir evenly, add 2% (g / ml) activated carbon and stir for 30 minutes, and decarbonize. Add disodium adenosine triphosphate, stir evenly, and prepare solution II for later use.
[0028] (c) Add an appropriate amount of water for injection into a stainless steel barrel, add calcium gluconate, low molecular weight dextran, and cysteine hydrochloride, add 2% (g / ml) activated carbon and stir for 30 minutes, decarbonize, and prepare solution III for later use.
[0029] (d) Pour the above thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com