Clinical grade cell protection solution and its preparation method and application
A technology for protecting liquid and cells, applied in the field of biomedicine, can solve the problems of inability to meet the requirements of long-distance use in different places, cannot be used directly for clinical use, and the cell viability decreases rapidly, so as to prolong the survival rate of cells and meet the requirements of long-distance use of cells in different places. , the effect of maintaining cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
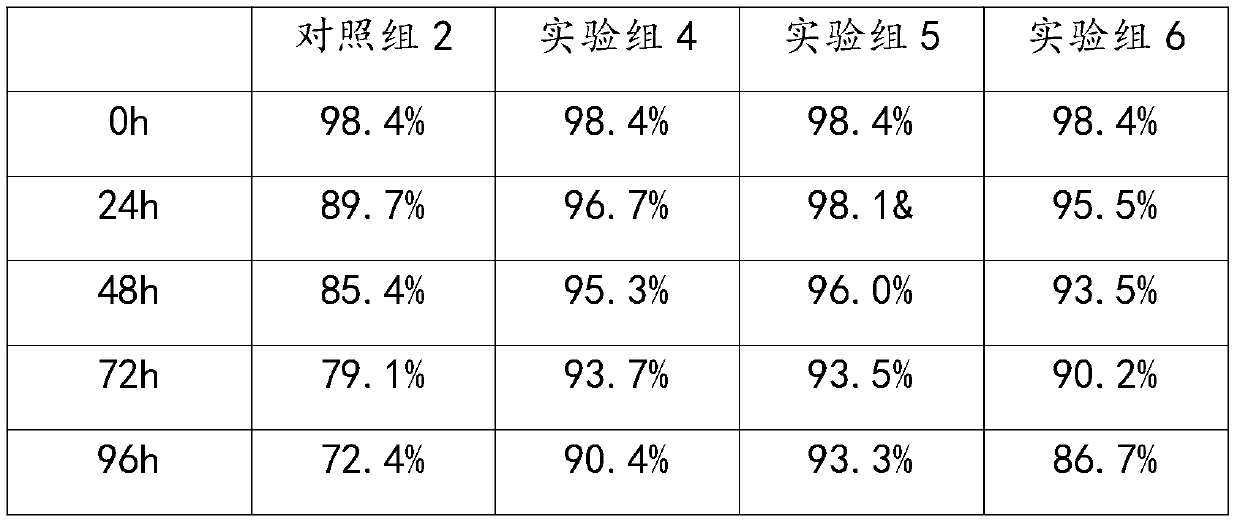
[0051] A clinical-grade cell protection solution, which contains human serum albumin injection, low-molecular dextran amino acid injection and isotonic sodium chloride injection, the distribution ratio of each component is: 7.5ml of human serum albumin injection, 3ml of Low molecular weight dextran amino acid injection and 89.5ml isotonic sodium chloride injection;
[0052] Among them, the low-molecular-weight dextran amino acid injection is preferably a compound preparation, and every 1000ml low-molecular-weight dextran amino acid injection contains water and the following components: 4.1g containing leucine, 1.8g isoleucine, 2.9g phenylpropanoid amino acid, 1.8g threonine, 2.0g valine, 0.6g tryptophan, 2.4g methionine, 3.4g glycine, 5.0g lysine, 2.2g arginine, 1.0g histidine, 60.0g dextran 40, the auxiliary material is sodium bisulfite;
[0053] The human serum albumin injection is preferably a pharmaceutical preparation, and its volume concentration of human serum albumin ...
Embodiment 2
[0057] A clinical-grade cell protection solution, which contains human serum albumin injection, low-molecular dextran amino acid injection and isotonic sodium chloride injection, the proportion of each component is: 5ml of human serum albumin injection, 5ml of low-molecular Molecular dextran amino acid injection and 90ml isotonic sodium chloride injection;
[0058] Among them, the low-molecular-weight dextran amino acid injection is preferably a compound preparation, and every 1000ml low-molecular-weight dextran amino acid injection contains water and the following components: 4.1g containing leucine, 1.8g isoleucine, 2.9g phenylpropanoid amino acid, 1.8g threonine, 2.0g valine, 0.6g tryptophan, 2.4g methionine, 3.4g glycine, 5.0g lysine, 2.2g arginine, 1.0g histidine, 60.0g dextran 40, the auxiliary material is sodium bisulfite;
[0059] The human serum albumin injection is preferably a pharmaceutical preparation, and its volume concentration of human serum albumin is 20%. ...
Embodiment 3
[0063] A clinical-grade cell protection solution, which contains human serum albumin injection, low-molecular dextran amino acid injection and isotonic sodium chloride injection. The distribution ratio of each component is: 2.5ml of human serum albumin injection, 8ml of Low molecular weight dextran amino acid injection and 89.5ml isotonic sodium chloride injection;
[0064] Among them, the low-molecular-weight dextran amino acid injection is preferably a compound preparation, and every 1000ml low-molecular-weight dextran amino acid injection contains water and the following components: 4.1g containing leucine, 1.8g isoleucine, 2.9g phenylpropanoid amino acid, 1.8g threonine, 2.0g valine, 0.6g tryptophan, 2.4g methionine, 3.4g glycine, 5.0g lysine, 2.2g arginine, 1.0g histidine, 60.0g dextran 40, the auxiliary material is sodium bisulfite;
[0065] The human serum albumin injection is preferably a pharmaceutical preparation, and its volume concentration of human serum albumin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com