Application of norisoboldine in preparing medicament for treating autoimmune disease
A technology of norisoboldine and autoimmunity, which is applied in the field of medicine and can solve the problems of no biological activity or clinical application of norisoboldine.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of norisoboldine: dry 3 kg of root tuber powder, reflux extraction with 95% ethanol for 3 times, 2 hours each time, concentrate under reduced pressure to obtain extract, add 2% hydrochloric acid to dissolve, and filter. The filtrate was chromatographed on a strongly acidic cation exchange resin column, washed with water, and the pH was adjusted to 8.5 with ammonia water, then the exchange resin was transferred to a Soxhlet extractor, and ether was refluxed for extraction. Ether was evaporated to obtain 4.8 g of dark brown extract. The extract was separated by silica gel column chromatography repeatedly, eluted repeatedly with chloroform-methanol (7:3) to obtain the crude compound I, and after repeated recrystallization, 0.76 g of brown powder Compound I was obtained. Co-silica gel thin layer chromatography with reference substance, R f The value is consistent, and the mixing melting point does not decrease, so the identification compound I is norisoboldine....
Embodiment 2
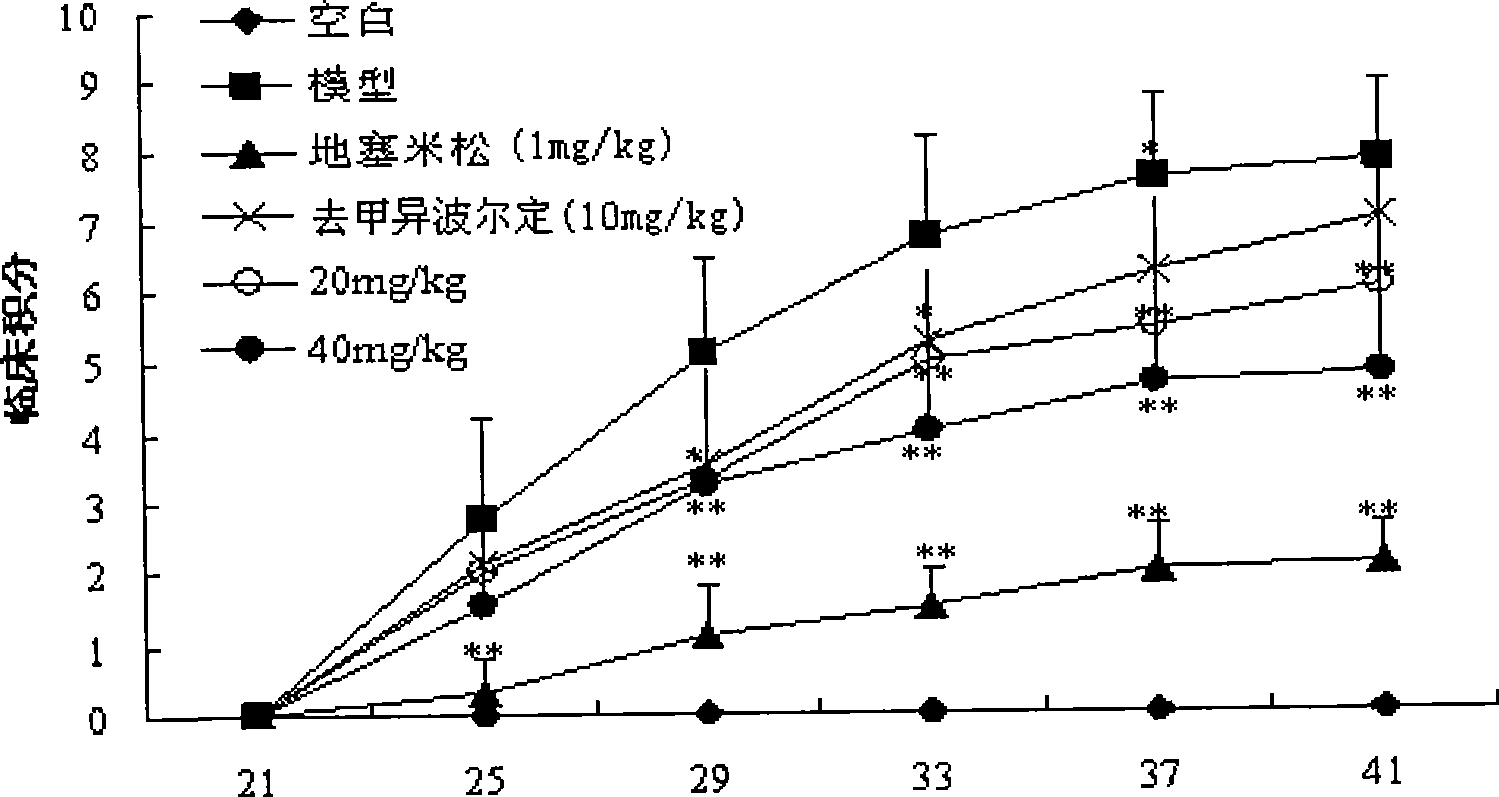
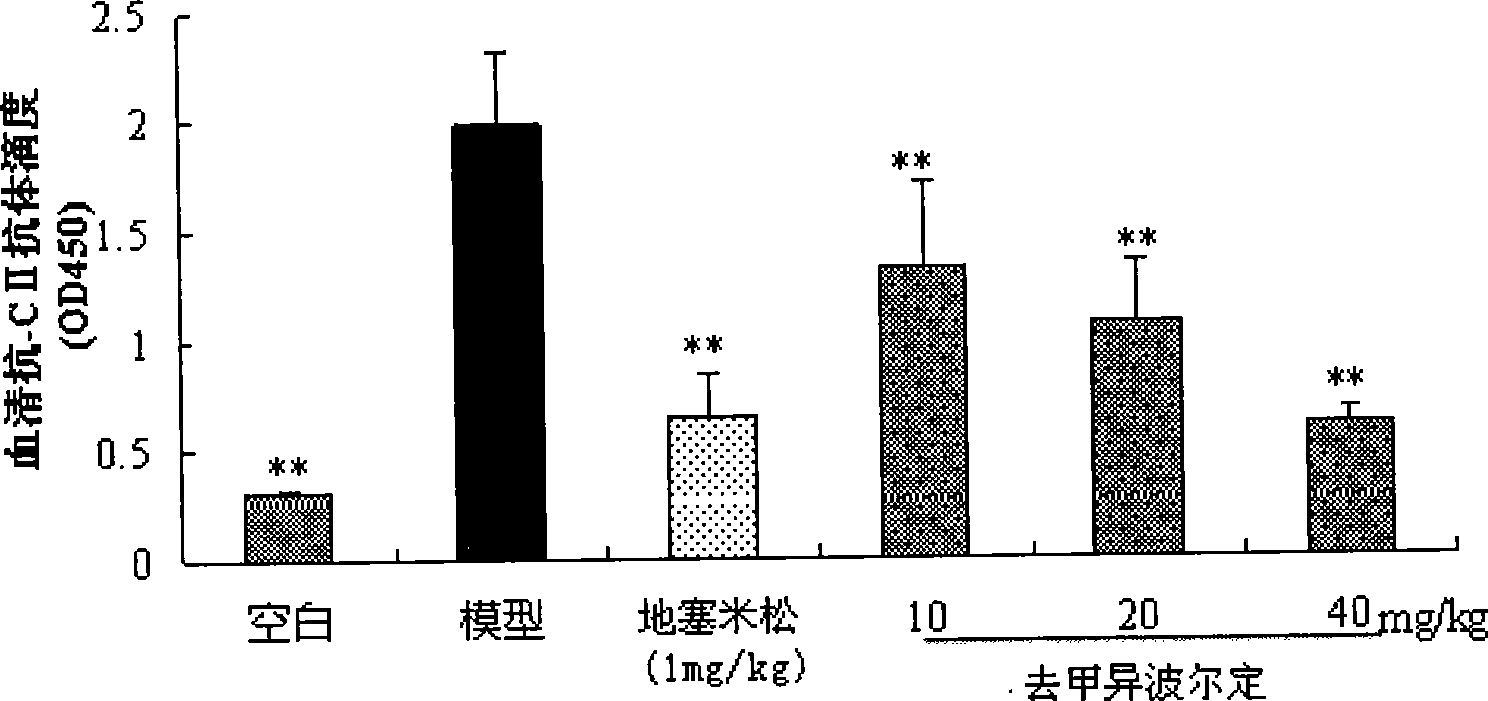
[0018] Effects of Noribordine on Type II Collagen-Induced Arthritis in Mice
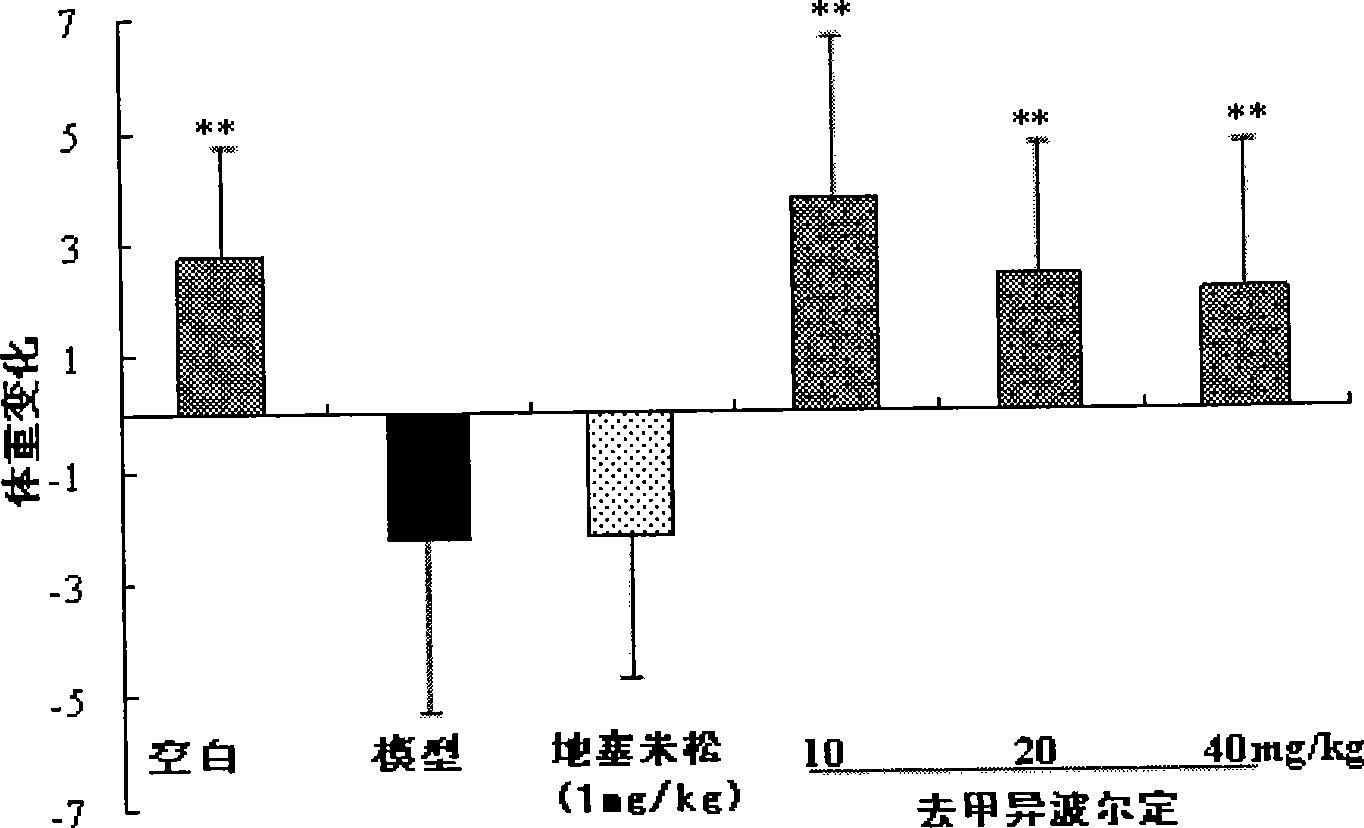
[0019] 1 The effect of noribordine on the body weight of mice with collagen arthritis
[0020] Add chicken type II collagen into 0.1M acetic acid, stir at 4°C until fully dissolved, and make the concentration 2mg / ml, overnight at 4°C, mix with an equal volume of complete Freund's adjuvant (containing 2mg / ml hot water) in an ice bath Inactivated Mycobacterium tuberculosis) were mixed and fully emulsified to obtain type II collagen emulsion. Male ICR mice were sensitized by intradermal injection of 100 μl type II collagen emulsion at the base of the tail, and the day of injection was recorded as d 0. On the 21st day, 100 μl of type II collagen emulsion was injected again at the same site to attack, and the mice were randomly divided into 6 groups (n=7-8): 1) normal group; 2) model group; 3) norisobolidine ( 10 mg / kg); 4) Noripordine (20 mg / kg); 5) Noripordine (40 mg / kg); 6) Dexamethasone (DEX, 1 mg / k...
Embodiment 3
[0038] Effect of Noribordine on Experimental Autoimmune Encephalomyelitis (EAE) in Rats
[0039] The guinea pig spinal cord was collected under aseptic conditions, made into a 50% homogenate, and fully mixed with the same amount of complete Freund's adjuvant to prepare the immunogen. Rats were injected with 0.1 mL of immunogenic emulsion in each of the two hind foot pads, and at the same time, subcutaneously injected pertussis vaccine (5.5×10 10 mL -1 ) 0.2 mL. The guinea pig spinal cord homogenate in the immunogen of the control group was replaced with an equal amount of normal saline, and the administration group was administered from the day of immunization for 18 consecutive days.
[0040] After immunization, the body weight of the rats was weighed every day, the condition was observed, and the neurological dysfunction score was performed. The scoring standard of neurological dysfunction adopts a 5-point scoring method: 0 points, no obvious abnormality; 1 point, tail we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com