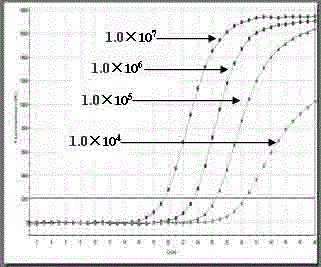
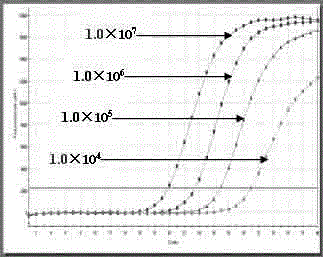
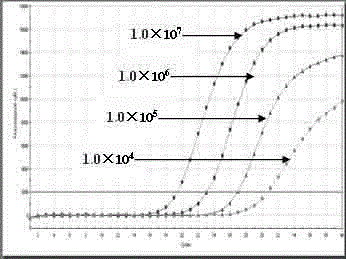
Single standard product-based four-color fluorogenic quantitative PCR (Polymerase Chain Reaction) method and kit
A technology of fluorescence quantification and standard products, which is applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problems of cumbersome operation and low detection sensitivity, and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1: Collection and delivery of specimens
[0119] The types of specimens suitable for testing include stool specimens from suspected patients, as well as specimens such as throat swab specimens, herpes fluid, cerebrospinal fluid, or virus isolation and culture.
[0120] Stool specimen collection and transportation methods: Swab feces or diarrhea with a sterile swab, put them in a sterile container, seal it and send it to the laboratory for testing immediately, and wrap the specimen in dry ice during transportation; or store it at -20°C for a short period of time. Long-term storage can be placed at -70°C, in principle, it should not exceed 6 months to prevent the degradation of viral RNA.
[0121] The method of collecting and transporting the throat swab specimens: swab the secretions from the posterior pharyngeal wall and tonsils on both sides of the throat swab under aseptic conditions, put them into the sampling tube, seal it and send it to the laboratory for t...
Embodiment 2
[0125] Embodiment 2: Extraction of viral RNA in the specimen
[0126] Take 100 μl of the sample solution collected above and add 300ul of the RNA extraction solution in the kit to fully shake, and place at room temperature for 5 minutes; add 100ul of chloroform, shake vigorously for 15 seconds, let stand at room temperature for 5 minutes, and centrifuge at 13,000rpm at 4°C for 10 minutes; carefully transfer the upper aqueous phase to Add an equal volume of isopropanol to a clean centrifuge tube, mix thoroughly, and centrifuge at 13,000rpm for 10min; discard the supernatant, add 500μl 75% DEPC ethanol, mix well, centrifuge at 13,000rpm for 10min, and carefully absorb most of the ethanol; The extraction tube was left open and dried in air at room temperature for 5 min until the ethanol evaporated, and the precipitate was dissolved with 20 μl DEPC H2O. Take 50 μl negative quality control product and add 300 μl Trizol RNA extraction solution to shake fully, let it stand at room te...
Embodiment 3
[0127] Example 3: Preparation of HRVA-NVGⅠ-NVGⅡ-HAstV single standard
[0128] 3.1 RT-PCR experiment, cDNA synthesis by reverse transcription
[0129] Using the above extracted RNA as a template, prepare a reverse transcription reaction system according to PrimeScript® 1st Strand cDNA Synthesis Kit (TAKARA) instructions, the total system is 20ul, and synthesize the first strand of cDNA:
[0130] Random primer 1.0ul dNTPs (10mM) 1.0ul RNA 5.0ul RNase free dH 2 o 3.0ul Total 10.0ul
[0131] The above reaction solution was cooled at 65°C for 5 minutes, and then cooled on ice.
[0132] The above reaction solution 10.0ul 5×PrimerScript Buffer 4.0ul RNase inhibitor (40U / ul) 0.5ul PrimerScipt Rtase (200U / ul) 1.0ul RNase free dH 2 o 4.5ul Total 20.0ul
[0133] 30°C, 10min; 42°C, 60min; 70°C, 15min, synthesize the first strand of cDNA, obtain HRVA, NVGⅠ, NVGⅡ and HAstV cDNA templates, and collect th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com