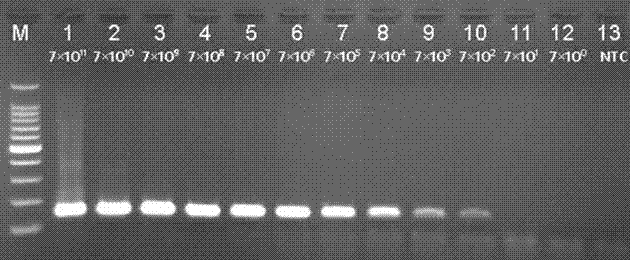
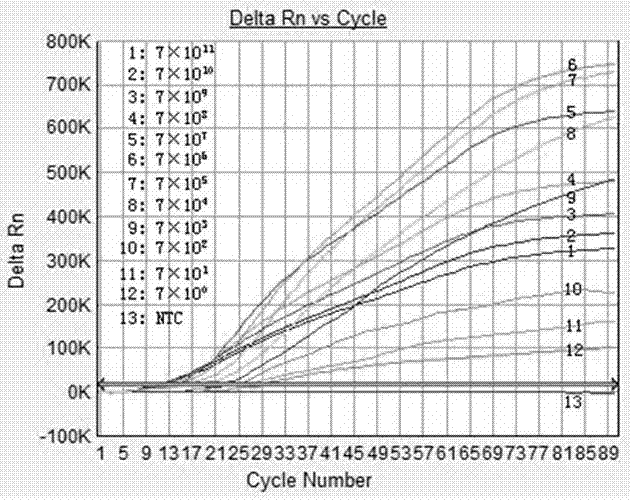
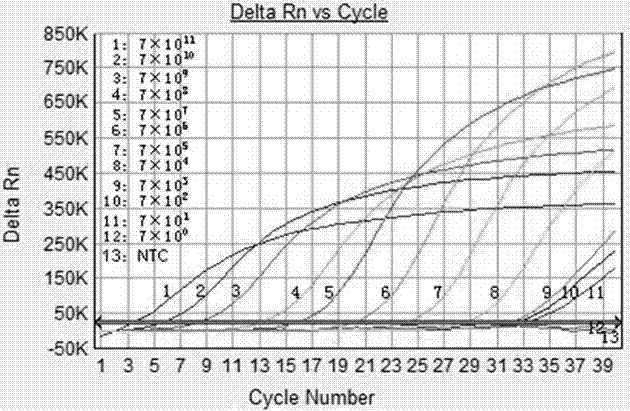
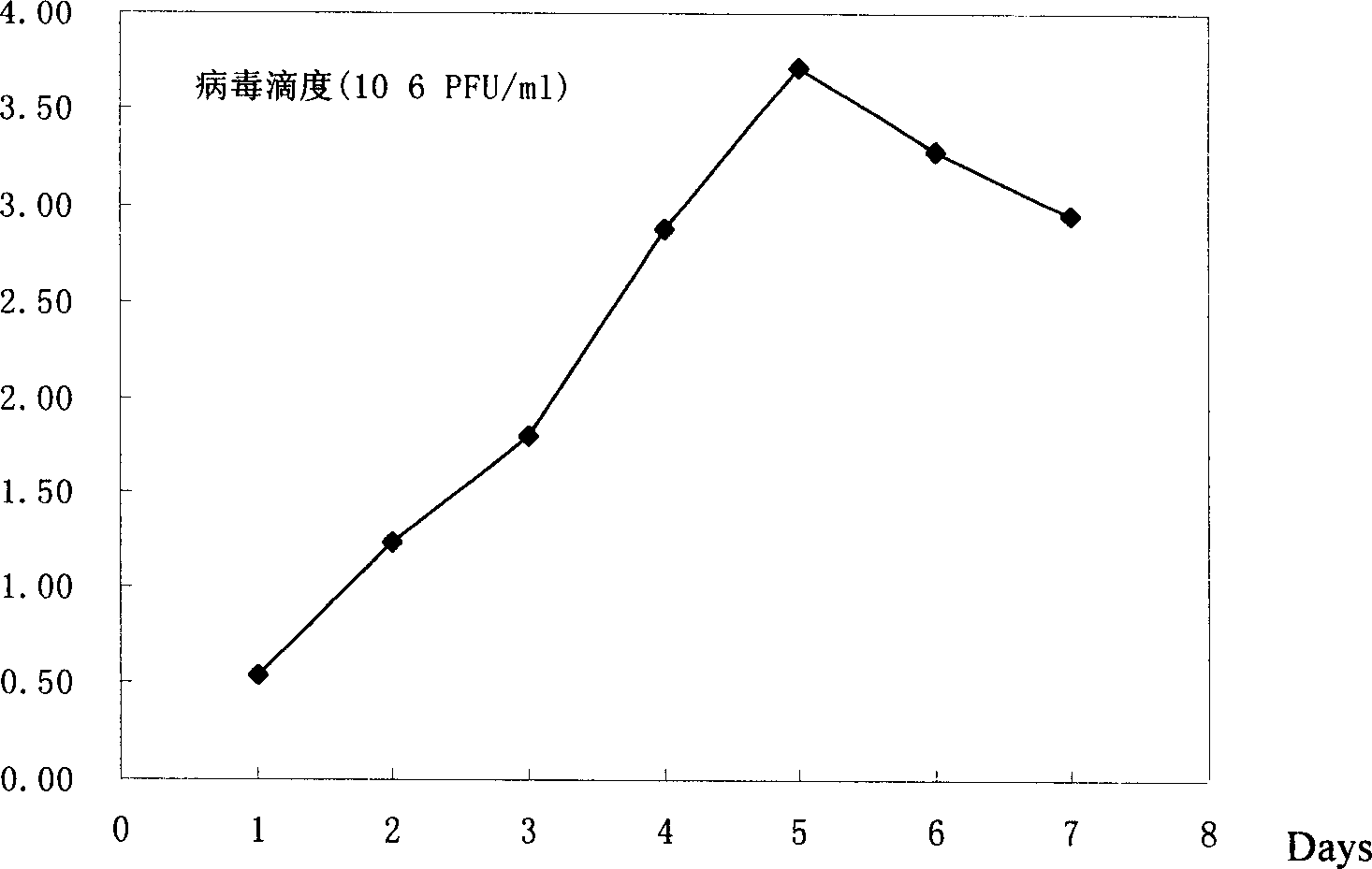
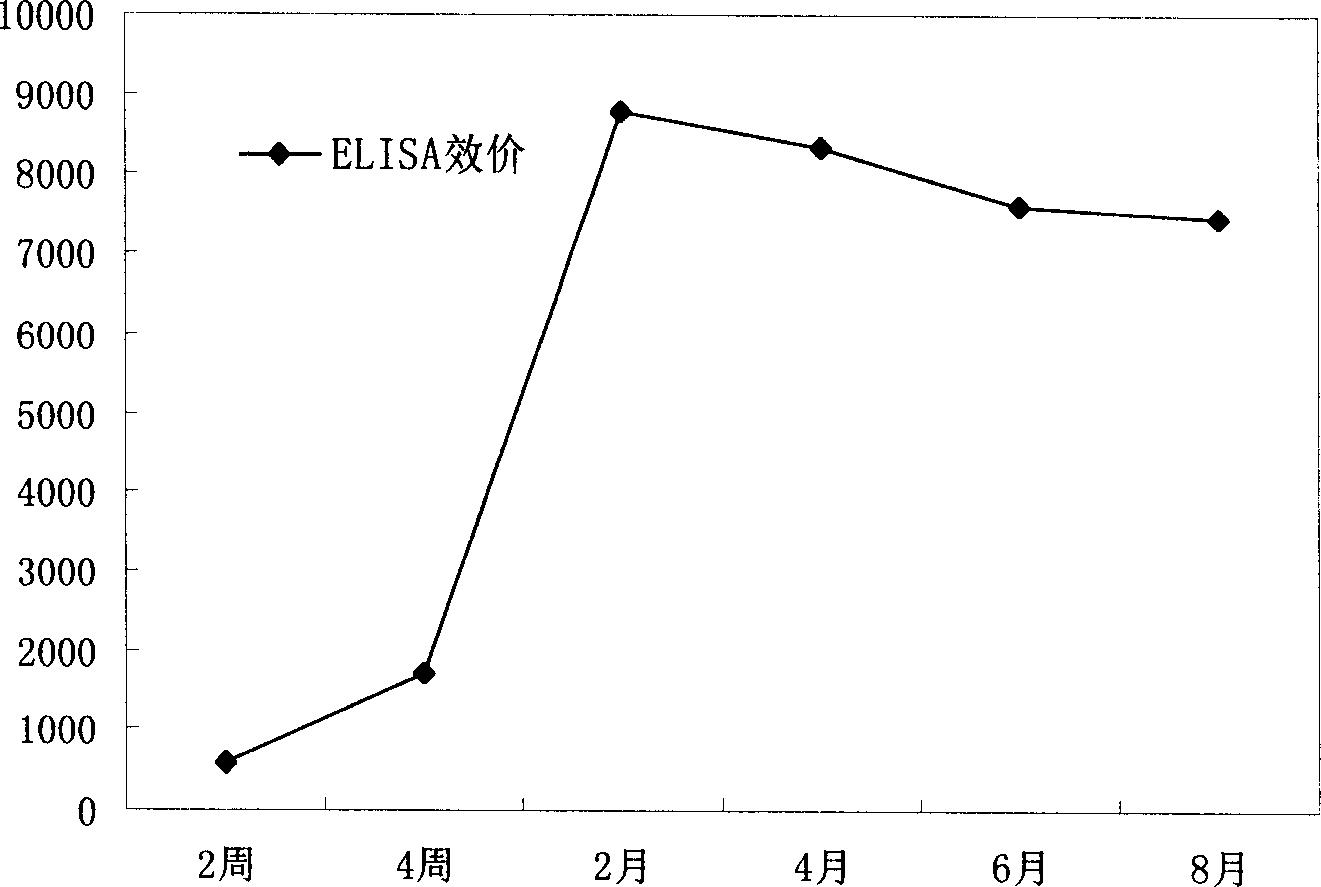
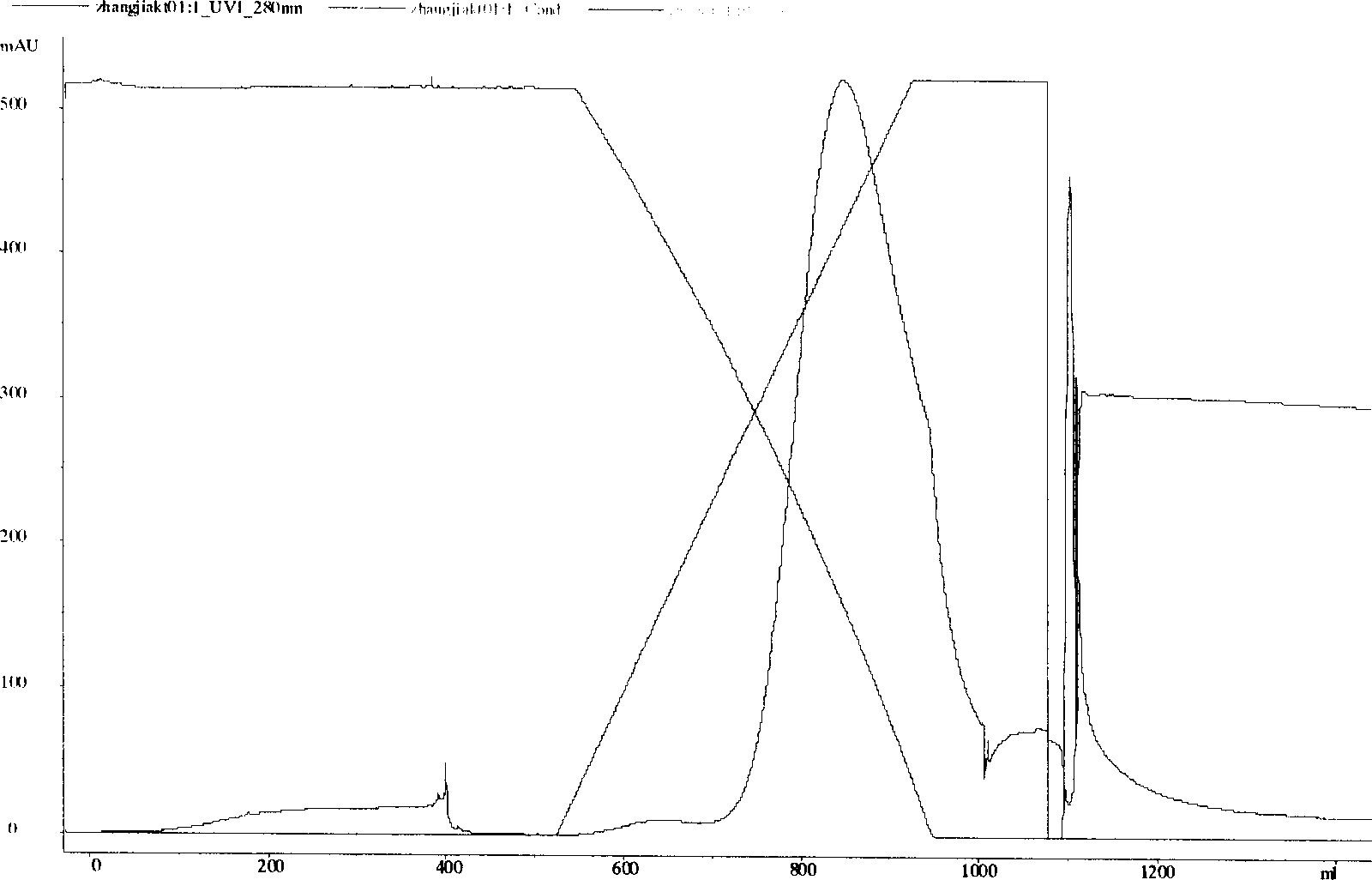
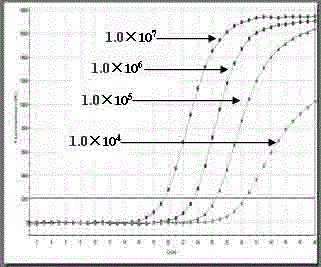
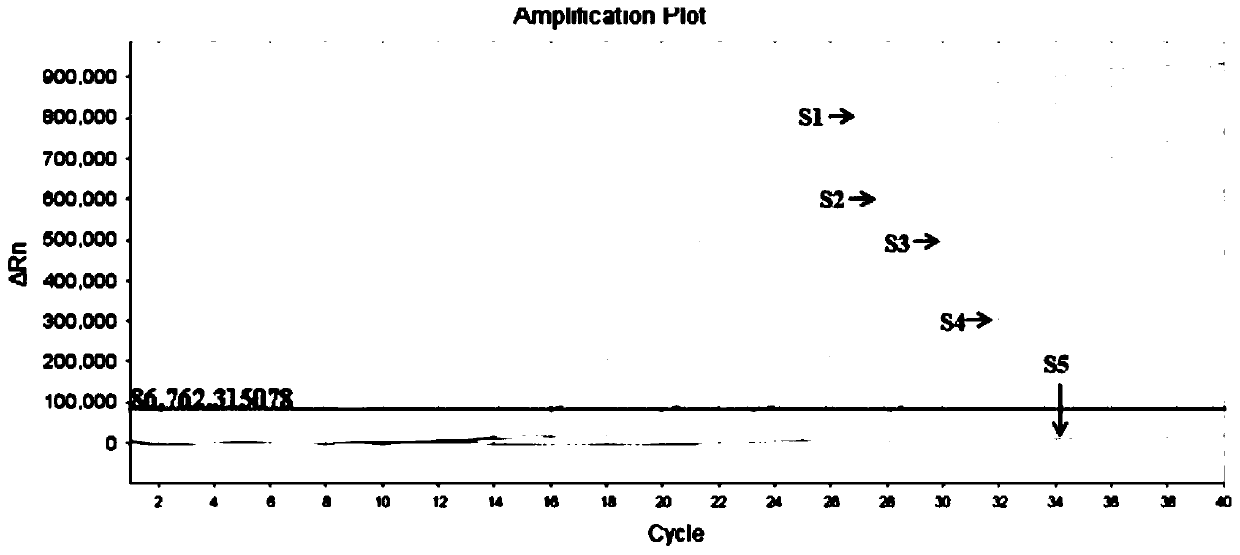
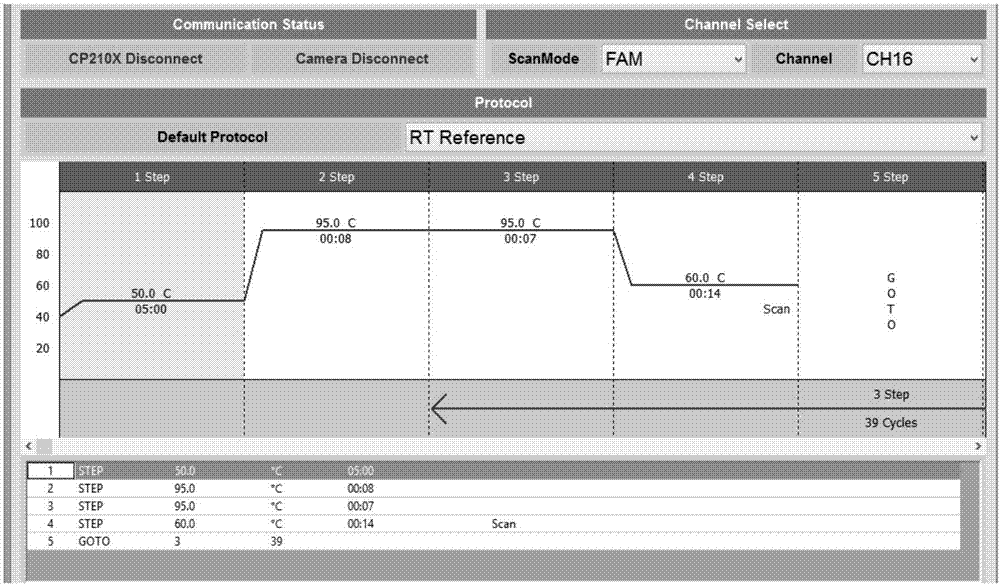
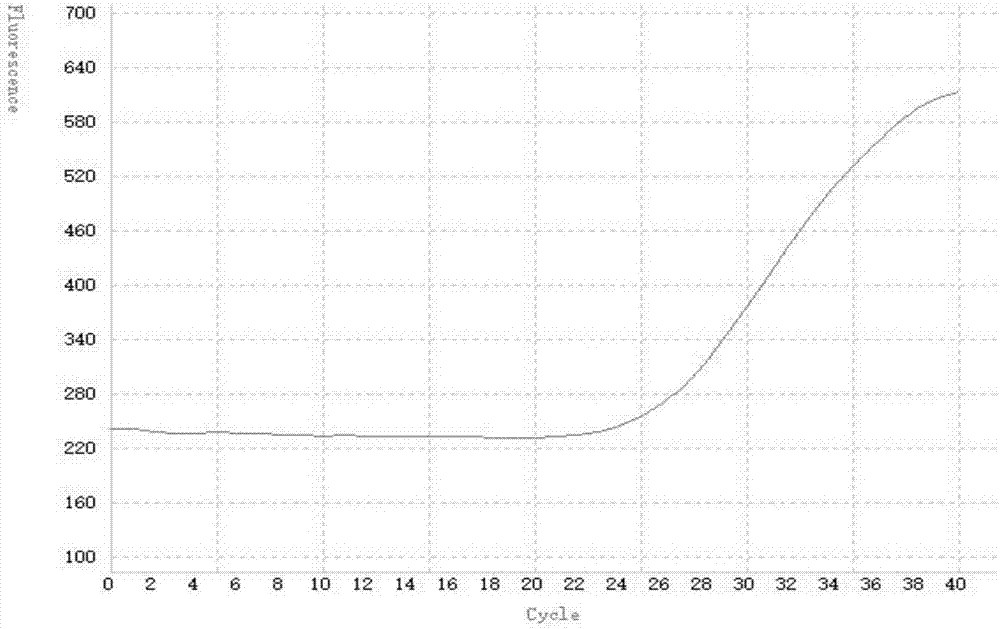
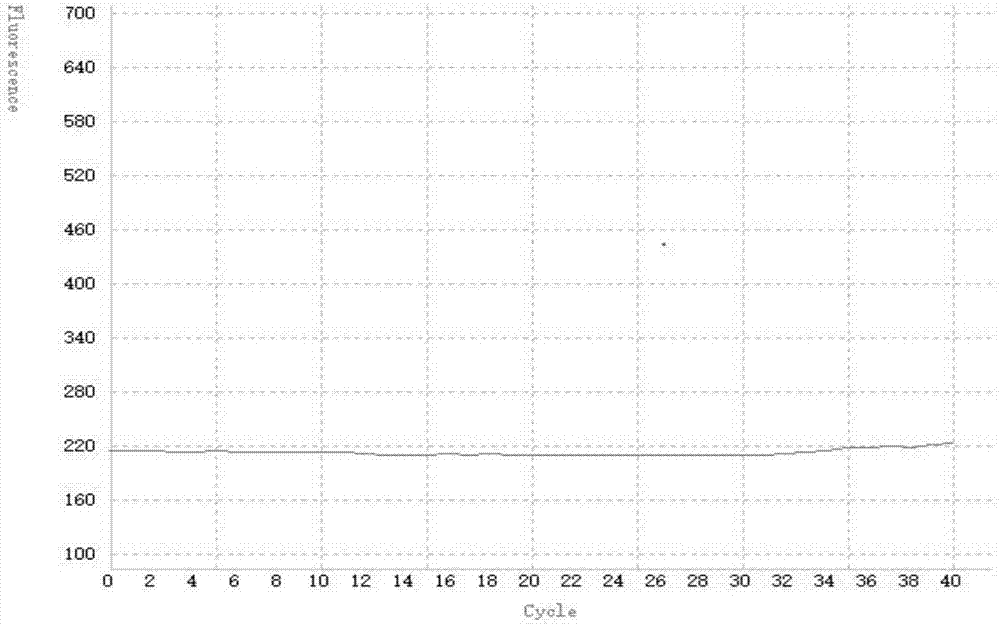
Patents
Literature
37 results about "Group A rotaviruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV
ActiveCN105154589AAvoid crossbreedingGuaranteed temperatureMicrobiological testing/measurementDNA/RNA fragmentationImmune profilingSwine Transmissible Gastroenteritis
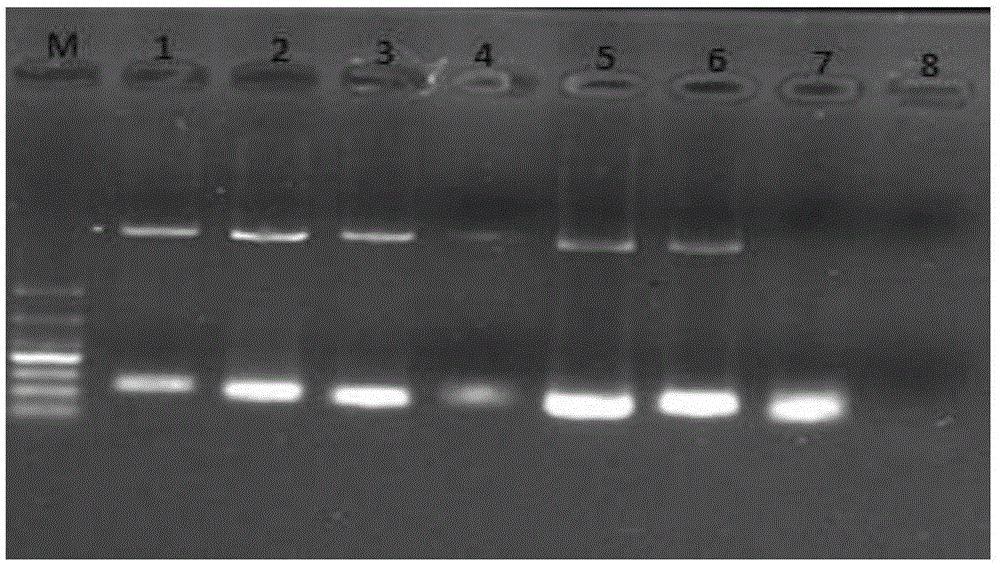
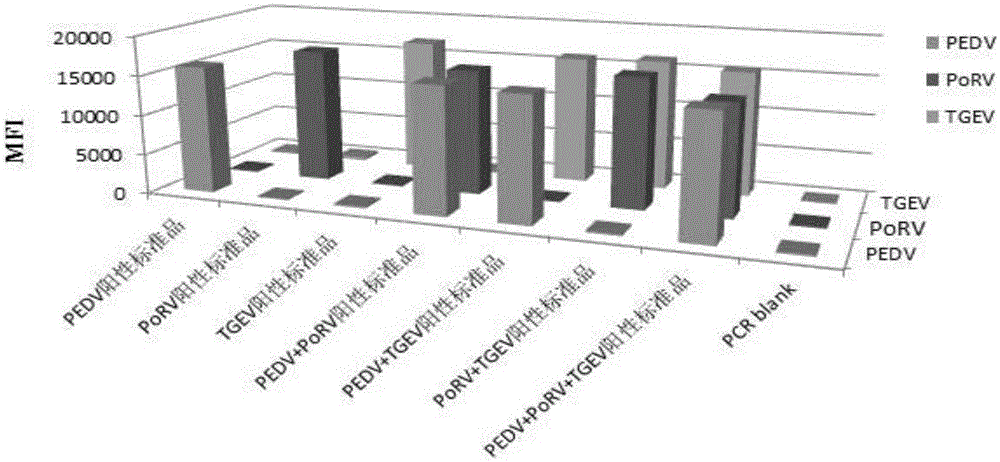
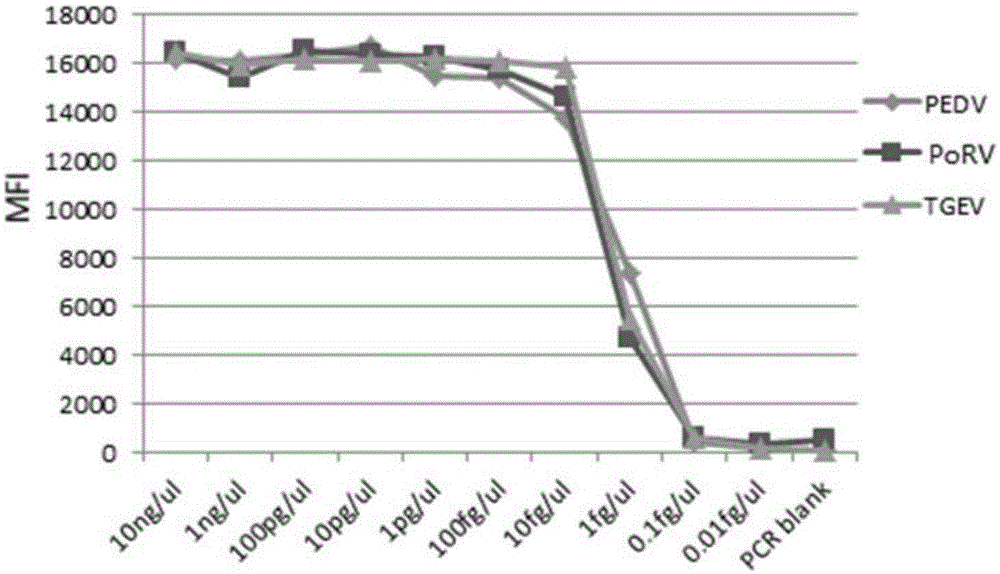
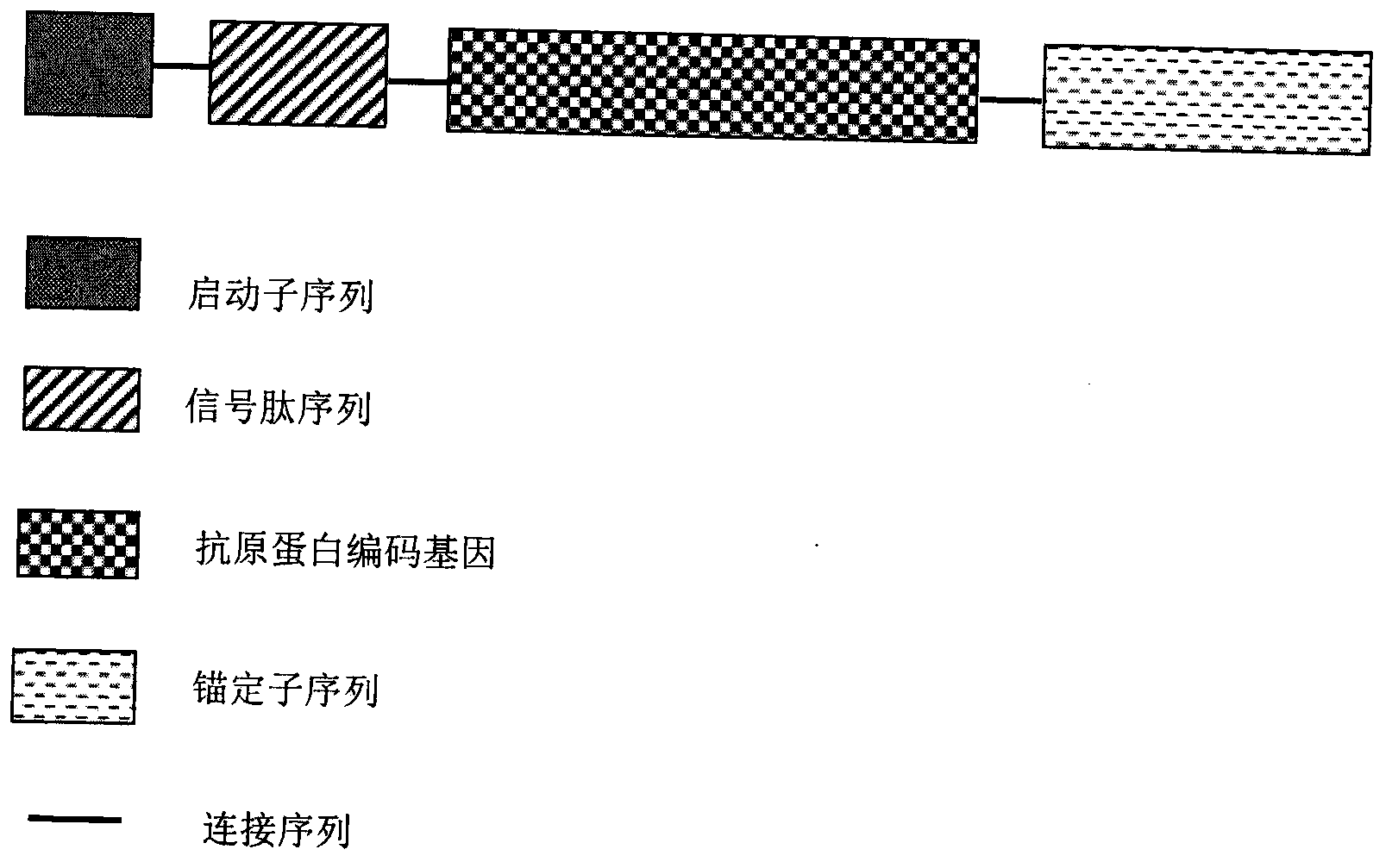
The invention discloses a multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV. The method is easy to operate, a target amplified fragment is obtained through PCR, then hybridization is conducted on an amplified product, fluorescence coded microspheres and streptavidin-phycoerythrin, the MFi value is read through a detection instrument, and different types of viruses are distinguished. By means of the method, porcine epizootic diarrhea, swine transmissible gastroenteritis and pig group A rotavirus can be accurately detected at the same time, the specificity is high, the sensitivity is high, and the repeatability is good. Compared with a traditional detection method, various molecules of different purposes in the same sample are detected at the same time, the sample consumption is little, operation is simple and fast, and the detection cost can be greatly lowered.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Food grade lactic acid bacteria active carrier Group A rotavirus vaccine and preparation method thereof
InactiveCN103656633AWill not spreadNo horizontal transferViral antigen ingredientsDigestive systemEscherichia coliSerotype
The invention discloses food grade lactic acid bacteria active carrier Group A rotavirus vaccine and a preparation method thereof. The food grade lactic acid bacteria active carrier Group A rotavirus vaccine is characterized in that VP6 antigen protein from Group A virus, common serotype VP7 antigen protein (P serotype) and VP4 antigen protein (G serotype-expressed separately in the form of VP5* and VP8* protein subunit), vaccine adjuvant escherichia coli thermal unstable toxin B (LTB) and cholera toxin subunit B (CTB) are expressed and secreted by thyA gene deletion lactic acid bacteria cell or shown by the cell wall. Expressions of antigen protein and vaccine adjuvant protein are controlled by inducible or constitutive promoter, protein expression cassette is integrated onto the chromosome of the expression host lactic acid bacteria strain, and external antibiotics resistance gene introduced in gene manipulation is removed. The lactic acid bacteria active carrier rotavirus vaccine has the advantages of having wide serotype covering range, being easy to produce in large scale, and being safe and convenient to use without a refrigerator and a needle tubing.
Owner:刘占良 +2
Group A rotavirus real-time isothermal amplification detection kit, primers and probe thereof
ActiveCN102965451AAvoid inconvenienceStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleic acid sequencing
The invention relates to an enteropathogen rapid detection technology based on real-time nucleic acid sequence-based amplification (NASBA). Specifically, the invention provides a group A rotavirus real-time isothermal amplification detection kit, a pair of primers and a molecular beacon probe. The kit includes: a 2*real-time NASBA reaction solution containing the primers and the probe, a 5*enzyme mixed solution and a positive control template, a negative control and a blank control. The sequences of the primers and the probe are the sequences numbered as SEQIDNO:1-3, and the primers and the probe can specifically amplify and detect group A rotavirus VP6 gene. The kit provided in the invention has the characteristics of fastness, high efficiency, sensitivity and specificity, and real-time detection analysis, etc., and can be used in the fields of conventional detection and disease control and prevention in clinical practice and ports.
Owner:SHENZHEN TOTAL TEST TECH
Yolk antirotavirus antibody preparation, method for preparing same and use thereof
InactiveCN1435260AHigh neutralizing activityPurification protocol technology is simple and practicalDigestive systemAntibody ingredientsYolkRotavirus RNA
Owner:上海海欣生物技术有限公司
Single standard product-based four-color fluorogenic quantitative PCR (Polymerase Chain Reaction) method and kit
InactiveCN103146846AEasy to operateQuick checkMicrobiological testing/measurementFluorescence/phosphorescencePcr methodDiagnosis laboratory
The invention discloses a single standard product-based quardruple four-color fluorogenic quantitative PCR (Polymerase Chain Reaction) technology. The technology is capable of detecting group A rotavirus (HRVA), norovirus group I (NVGI), norovirus group II (NVG II) and human astrovirus (HAstV) RNA in various treated specimen through one-step quardruple PCR amplification and quantifying virus load. The technology is characterized in that single standard product is adopted as positive control and quantitative standard in the quardruple four-color fluorogenic quantitative PCR detection for four viruses, thereby breaking through the deficiency that the traditional multiple fluorogenic quantitative PCR needs a plurality of standard products, improving the experiment detection efficiency, reducing the pollution probability and improving the preparation and production efficiency of the kit. The method and kit can be used for fast high-throughput test and epidemic monitoring in laboratories of HRVA, NVG I, NVG II and HAstV which can cause diarrhea syndromes. The single standard product-based four-color florogenic quantitative PCR method and the kit belong to the biotechnology field.
Owner:GUANGZHOU VIPOTION BIOTECH
Kit and detection method for accurate quantitative detection of group A rotaviruses
ActiveCN106222300AImprove tolerancePrevent deviationMicrobiological testing/measurementMicroorganism based processesMicrobiologyNucleotide sequencing
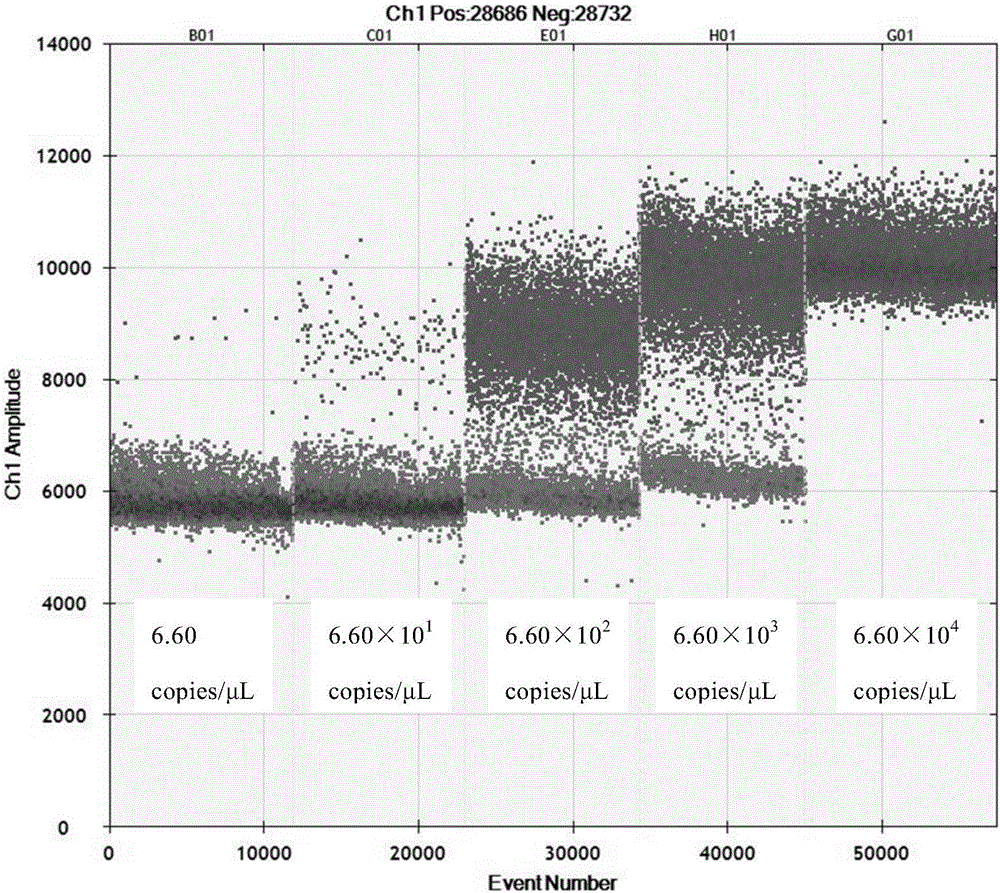
The invention discloses a kit and detection method for accurate quantitative detection of group A rotaviruses, and particularly relates to a set of primers and probes having nucleotide sequences shown in sequence tables of SEQ ID No.1 to SEQ ID No.3 used for detecting the group A rotaviruses, and the kit containing the primers and probes and the detection method for the group A rotaviruses. The detection method for the accurate quantitative detection of the group A rotaviruses by using a droplet digital PCR (Polymerase Chain Reaction) technology provided by the invention has higher sensitivity, accuracy and repeatability, and is easily standardized.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
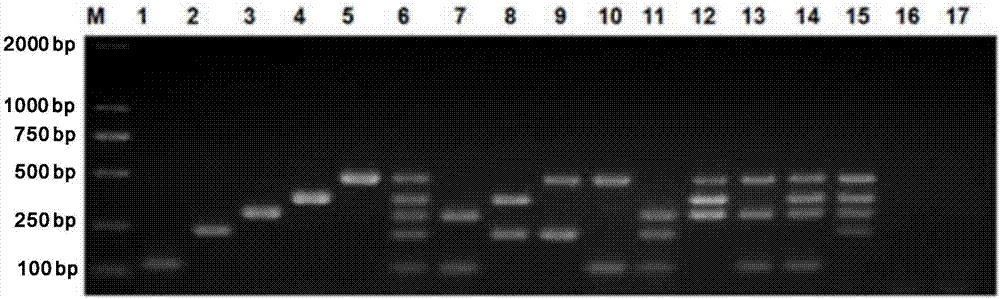
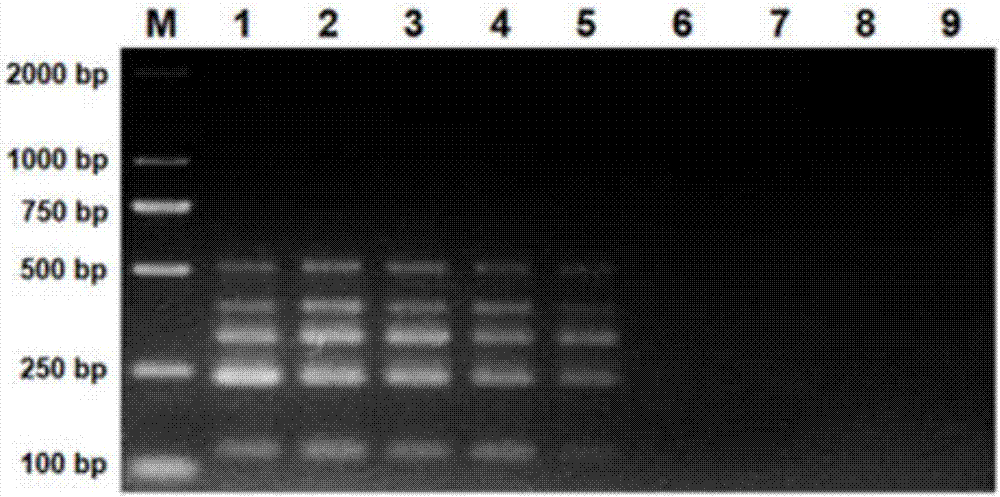
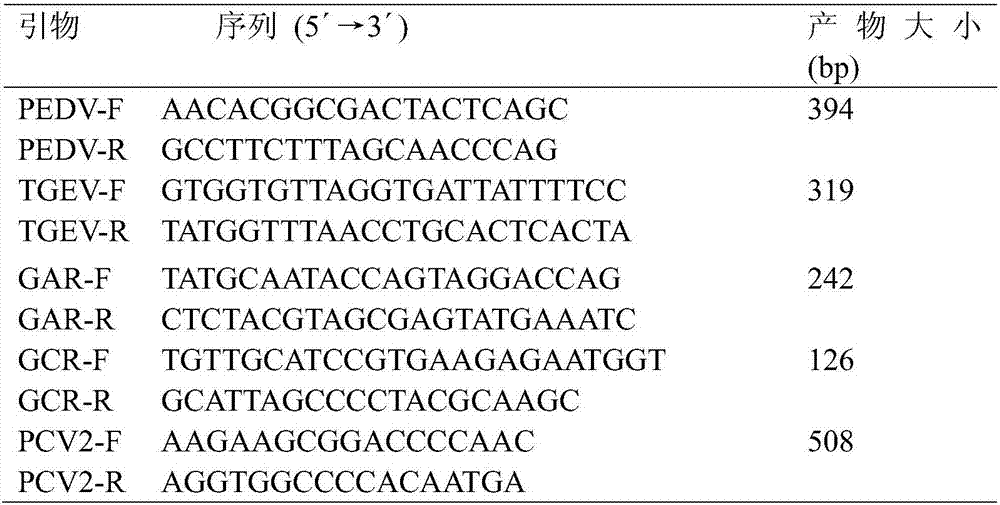
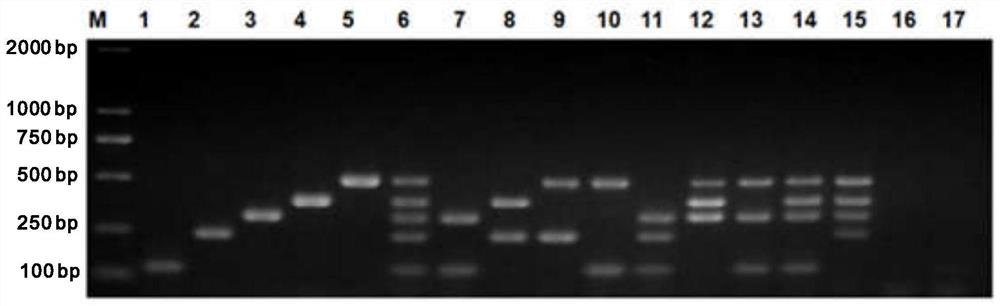
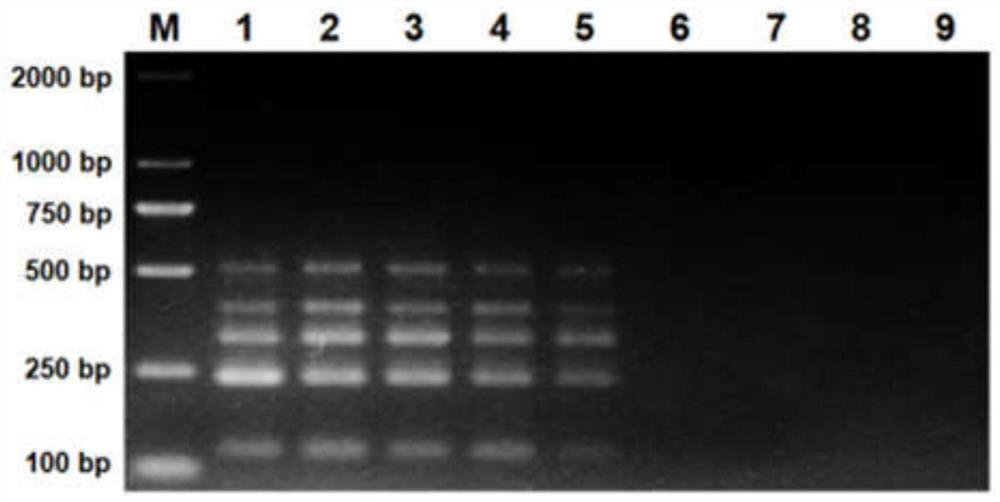
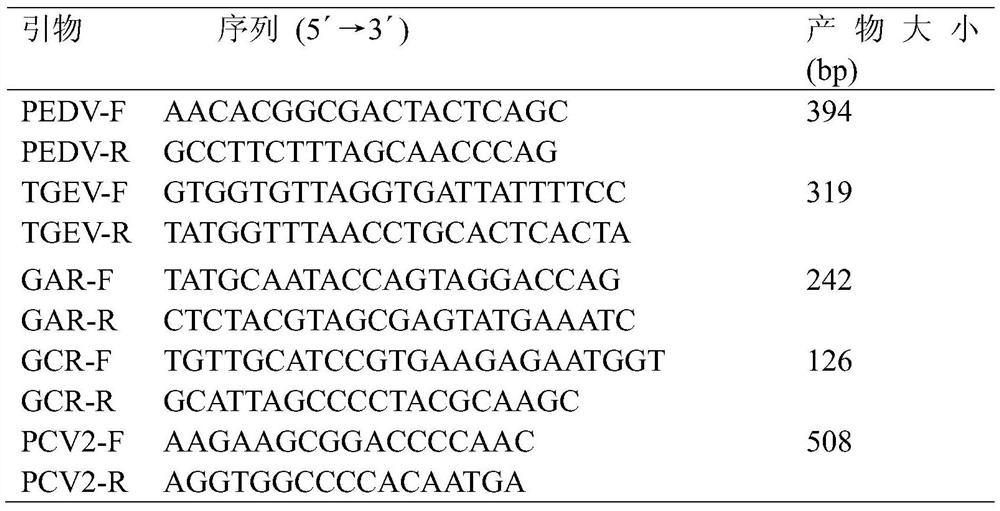
Multi-PCR (polymerase chain reaction) rapid diagnostic kit for five porcine diarrhea viruses and application of the multiple PCR rapid diagnostic kit
ActiveCN107419034AReduce mortalityReduce economic lossMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlPorcine circovirus
The invention discloses a multi-PCR (polymerase chain reaction) rapid diagnostic kit for five porcine diarrhea viruses. The multi-PCR rapid diagnostic kit comprises five pairs of PCR primers, PCR reaction liquid, a PCR standard substance, a positive control substance and a negative control substance. The invention further discloses application of the kit in simultaneous specific detection of infection of the five porcine diarrhea viruses. The five porcine diarrhea viruses include TGEV (transmissible gastroenteritis virus), GAR (porcine group A rotaviruses), GCR (porcine group C rotaviruses), PEDV (porcine epidemic diarrhea virus) and PCV2 (porcine circovirus 2).
Owner:杭州洪桥中科基因技术有限公司 +1
Composition, kit and method for detecting rotavirus group A
ActiveCN102260752AAccelerated deathBiohazard ReductionMicrobiological testing/measurementFluorescence/phosphorescenceMicrobiologyGenome
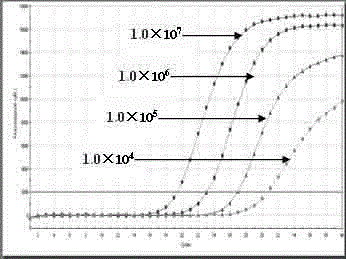
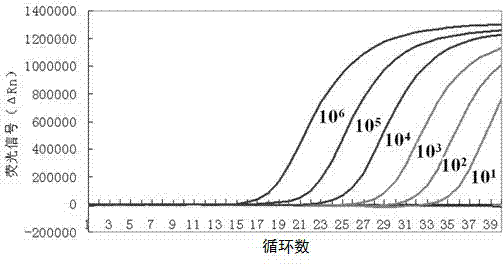
The invention discloses a composition for detecting group A rotavirus, belonging to the technical field of biology, comprising the following primers: 5'-GGCTTTAAAAGAGAGAATTTCC-3' and 5'-AAYTGYGGTATATTCAATACCATACA-3', also comprising the following probes: 5'-fluorescent reporter dye AARGAGCTAWCCGYTAGCCAGACGG-fluorescence quencher-3'. The invention further discloses a kit containing the primers andprobes and a method for detecting group A rotavirus. When using the composition, the kit and the / or method to detect group A rotavirus, a genome equivalent / reaction system with the range of 10<8>-10<1> copies is detected, the detection is rapid and simple, and the whole detection process can be completed within 3 hours with high specificity and high accuracy.
Owner:成都新基因格生物科技有限公司
Isothermal nucleic acid amplification system with high specificity as well as application thereof
ActiveCN108754032AAccurate detectionStrong specificityMicrobiological testing/measurementFluorescenceBiology
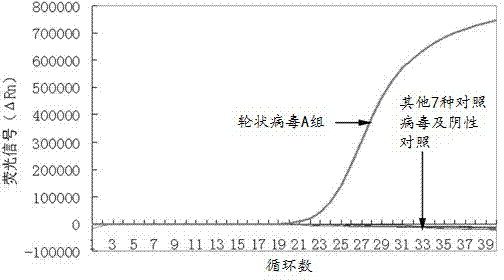
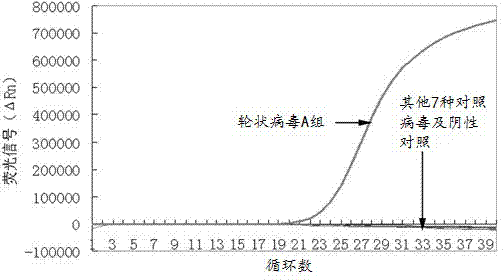
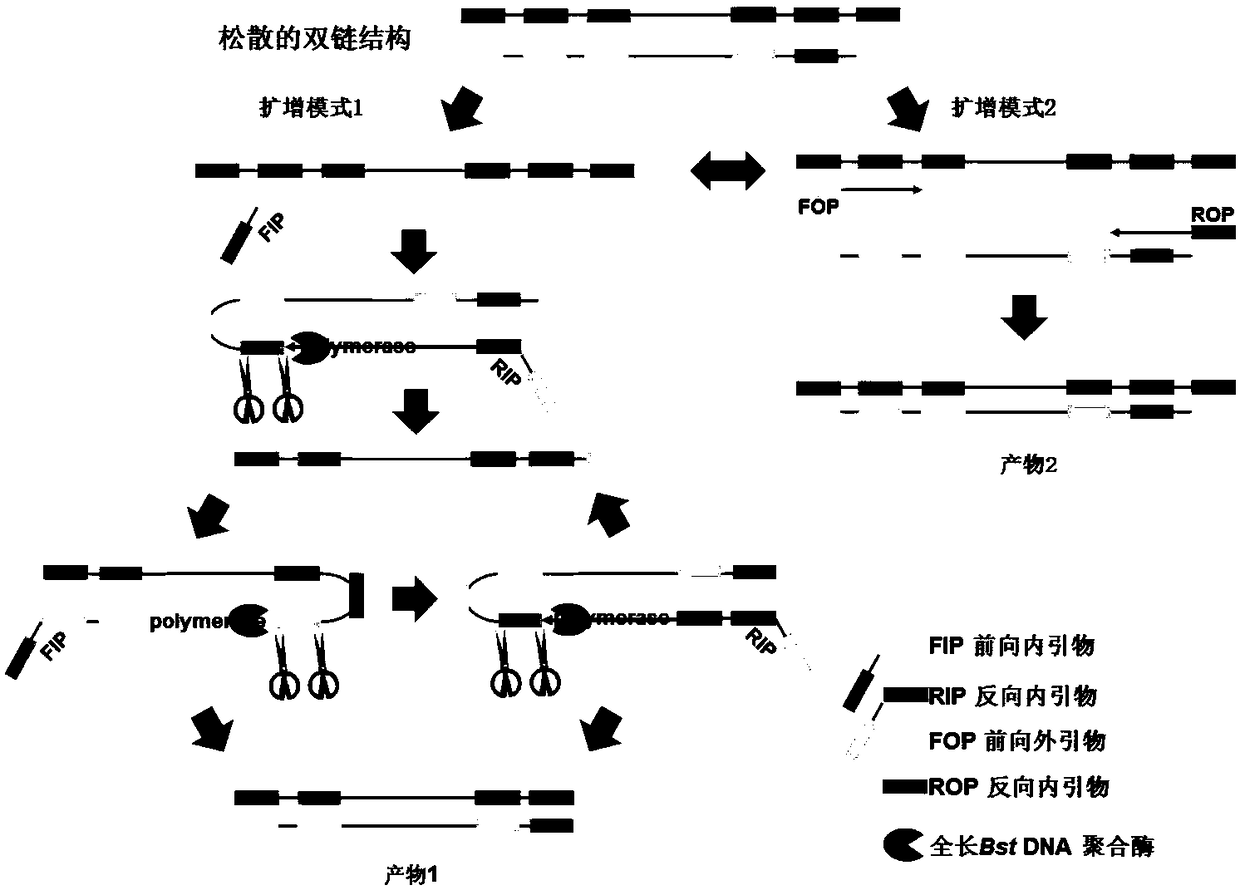
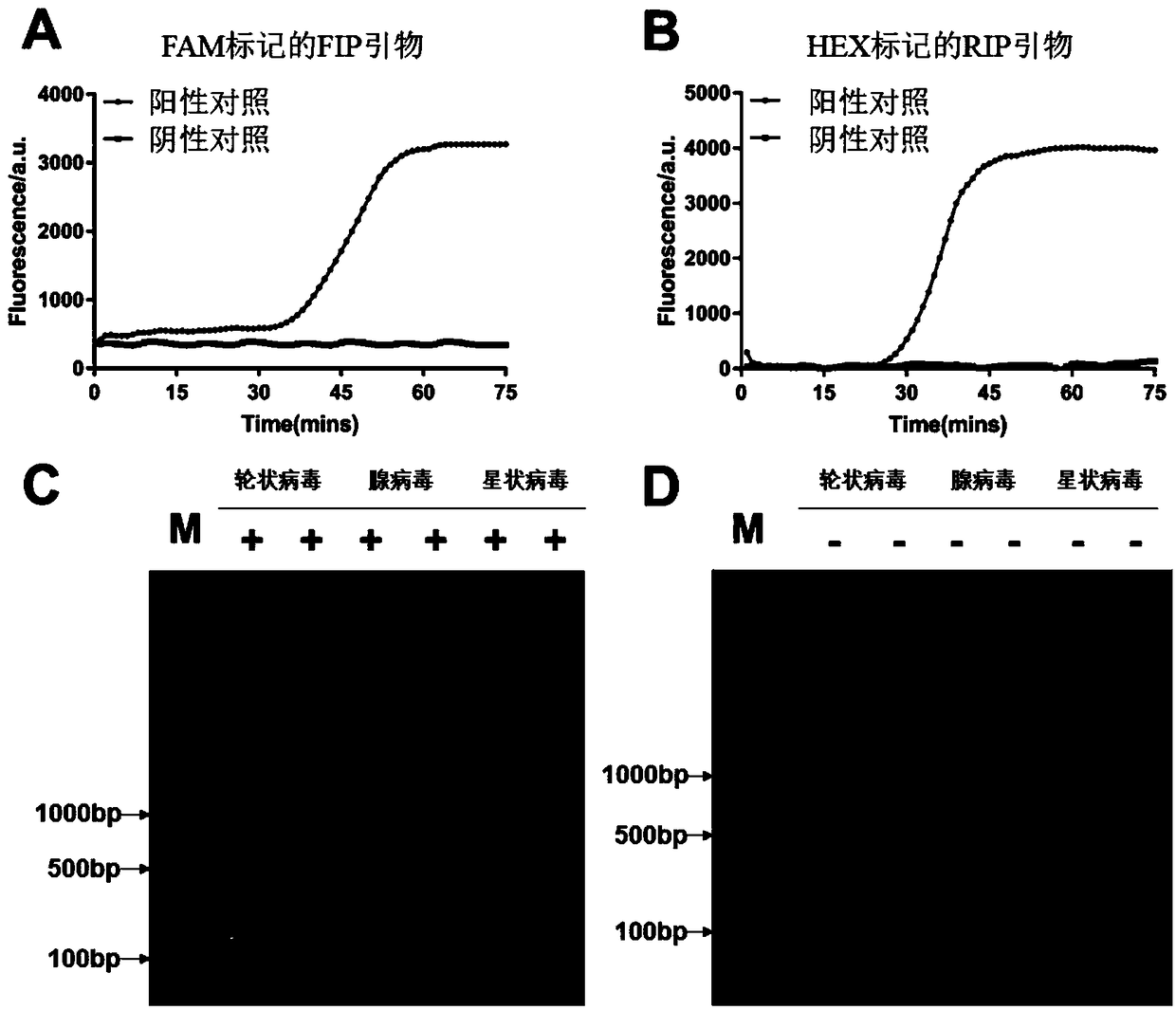
The invention relates to an isothermal nucleic acid amplification system with high specificity. The isothermal nucleic acid amplification system with high specificity comprises overall-length Bst DNApolymerase and also comprises embedded fluorescent dye. The invention also relates to a kit containing the isothermal nucleic acid amplification system as well as the application of the isothermal nucleic acid amplification system to childhood diarrhea pathogen detection. In the application, amplification primers comprise primer groups which are as shown in SEQ ID NO.1 to SEQ ID NO.4, SEQ ID NO.5to SEQ ID NO.8 and SEQ ID NO.9 to SEQ ID NO.12 and are correspondingly used for detecting Group A rotaviruses, astroviruses and adenoviruses. The overall-length Bst DNA polymerase has 5'-3'-duplex-specific excision enzyme activity and polymerization activity, the characteristic is applied to the Exo-NAT method of the invention, the embedded fluorescent dye is added into the reaction system and melting curve analysis is cooperated, so that high-specificity and high-sensitivity multiple nucleic acid detection can be realized; and the isothermal nucleic acid amplification system is successfully applied to accurate detection of childhood diarrhea pathogens, and has an important clinical significance and a wide application prospect.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD
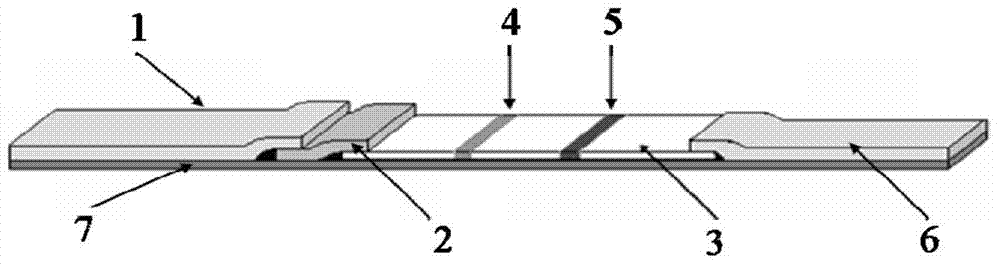
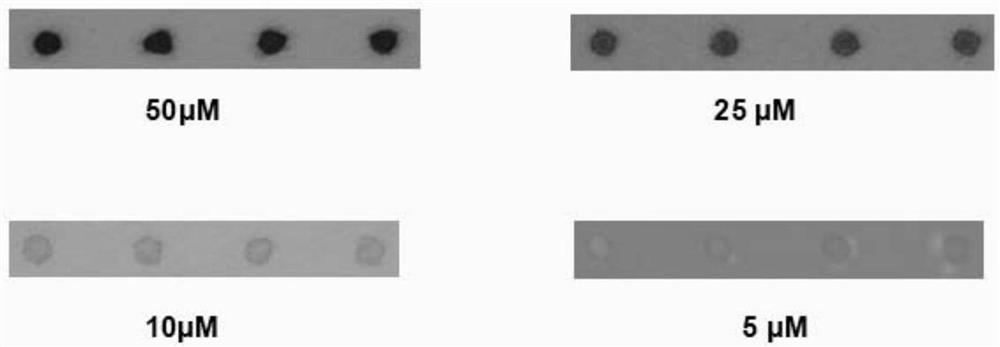
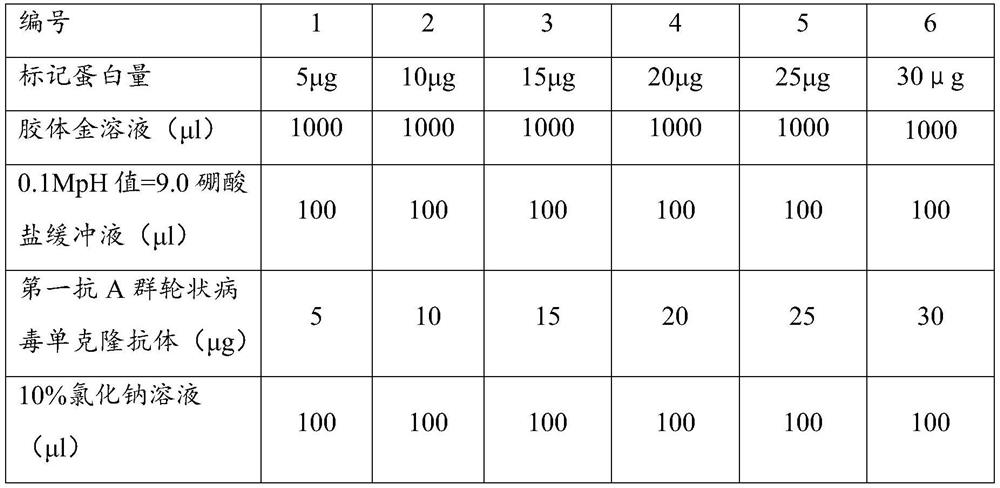
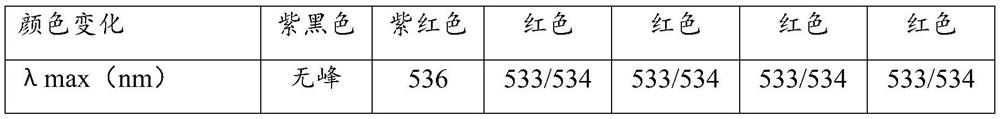
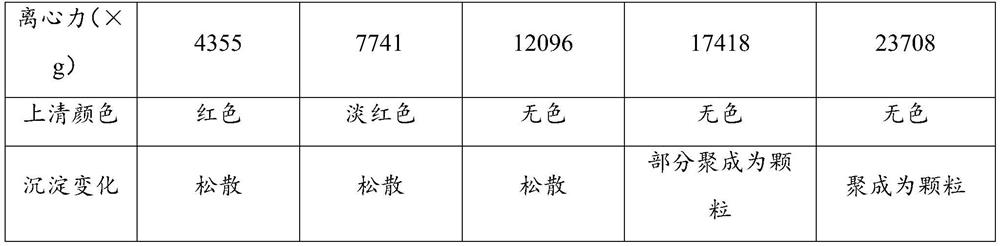
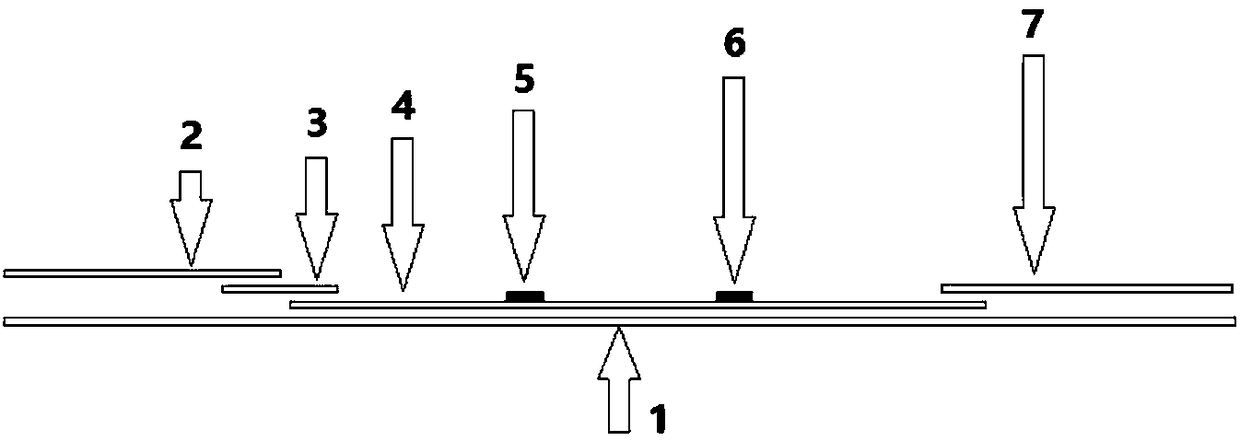
Immune test paper for detecting group A rotaviruses, and its making method
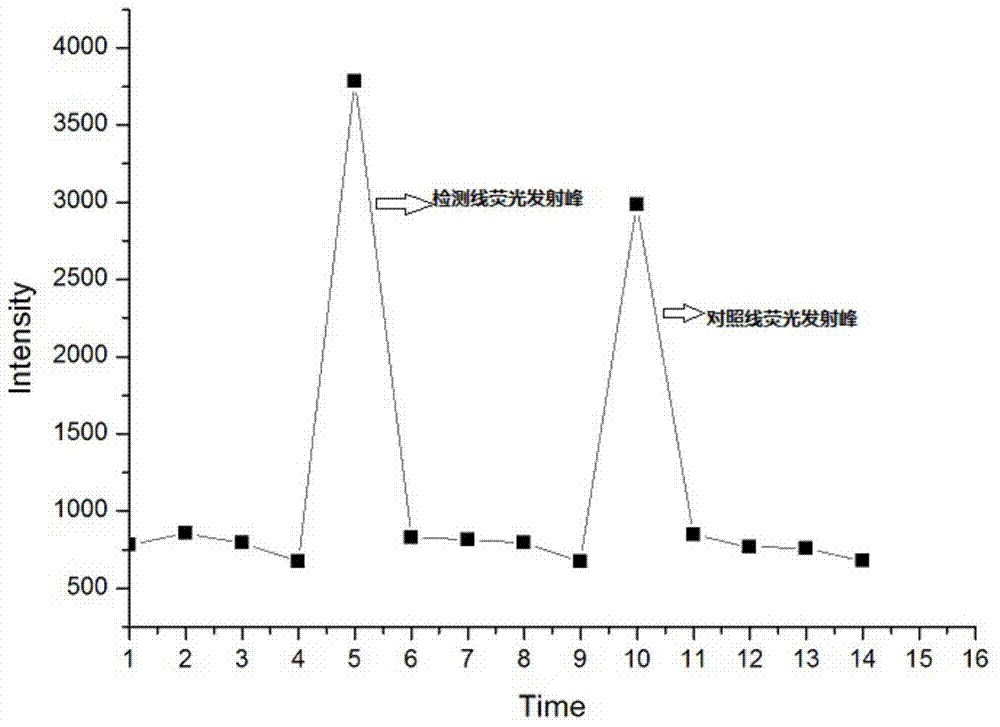
An immune test paper for detecting group A rotaviruses comprises a sample pad, a bonding pad, a cellulose nitrate film, a water absorbing pad and a back lining, the sample pad, the bonding pad, the cellulose nitrate film and the water absorbing pad are boned on the back lining, two ends of the bonding pad have a lapping joint with the sample pad and the cellulose nitrate film respectively, one end of the cellulose nitrate film far from the binding pad has a lapping joint with the water absorbing pad, the cellulose nitrate film is provided with a detection line and a quality control line which are spaced, the bonding pad is provided with a group A porcine rotavirus monoclonal antibody A-fluorescent marker, the detection line is formed by a group A monoclonal porcine rotavirus antibody B, and the quality control line is formed by goat anti-mouse IgG. The invention also provides a making method of the immune test paper for detecting group A rotaviruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Method for simultaneously detecting three groups of rotaviruses A, B and C
InactiveCN110714097AImprove detection accuracyStrong specificityMicrobiological testing/measurementMicroorganism based processesEpidemiologic studyMicrobiology
The invention discloses a method for simultaneously detecting three groups of rotaviruses A, B and C, and belongs to the field of biological detection. A pair of specific primers and a specific probewith different fluorescent labels are designed for target genes of group A rotavirus, group B rotavirus and group C rotavirus, a pair of universal primers is further designed, and during PCR amplification, the specific primers with universal primer tags are used for enrichment and amplification; and then universal primer tags are used for exponential amplification, and fluorescence signals of exponential amplification products are collected for detection. The method can simultaneously detect the group A rotavirus, the group B rotavirus, and the group C rotavirus. The method is high in specificity, high in sensitivity and short in detection time, and can be used for early rapid diagnosis of rotavirus infection and epidemiological study of the rotavirus.
Owner:山东凯景生物技术有限公司
Group-A rotavirus rapid real-time fluorescent RT-PCR (reverse transcription-polymerase chain reaction) detection kit
InactiveCN107502681AAvoid false negativesAvoid false positivesMicrobiological testing/measurementDNA/RNA fragmentationForward primerFluorescence
The invention discloses a group-A rotavirus rapid real-time fluorescent RT-PCR (reverse transcription-polymerase chain reaction) detection kit which comprises an amplification primer, a forward primer 5'-gttgatgctcaagatggagt-3', a reverse primer 5'-acttcattgtaatcatattgaatacc-3' and a specific fluorescent probe 5'-X-cagcaacaactgcagcttcaaaagaagtgt-Y-3'. The kit can meet the requirement of timeliness for group-A rotavirus detection, group-A rotaviruses are rapidly and quantitatively detected in real time according to one-step fluorescent RT-PCR technology by the design of the primers, the probe and reaction conditions in a reaction system and heat-resisting DNA (deoxyribonucleic acid) rapid polymerase, and sensitivity can reach 10<2> PFU / ml.
Owner:NANTONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF THE PEOPLES REPUBLIC OF CHINA
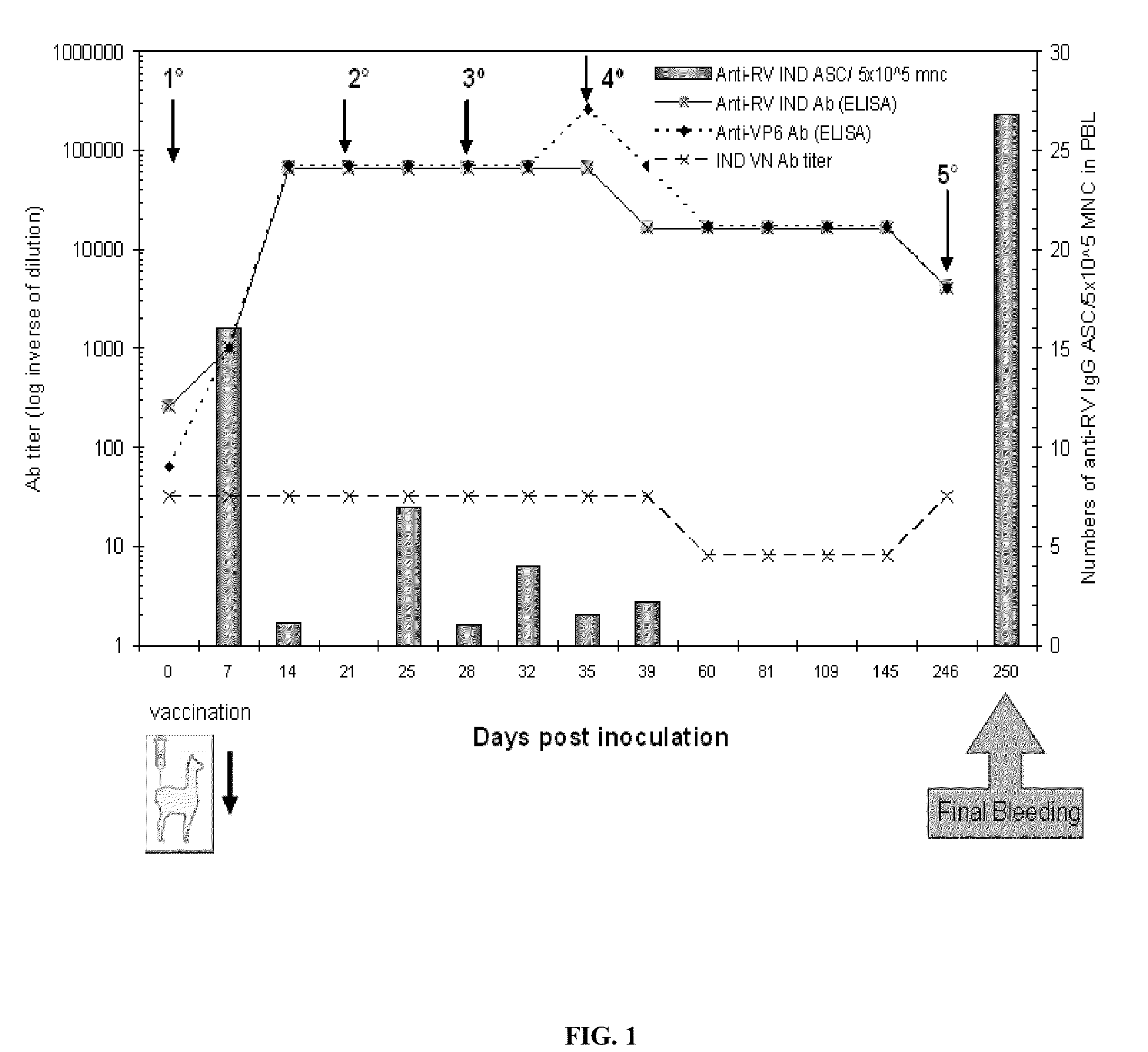
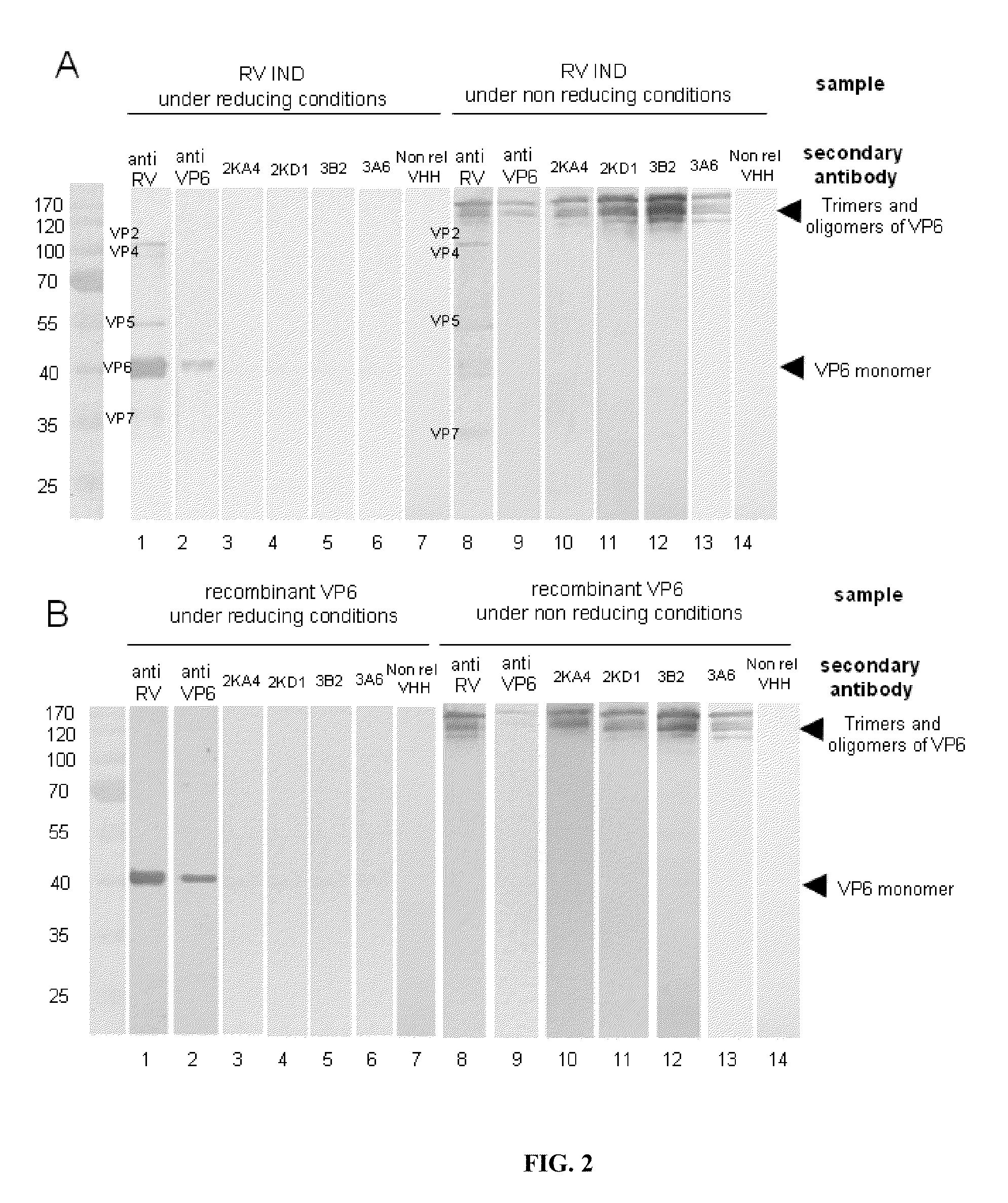
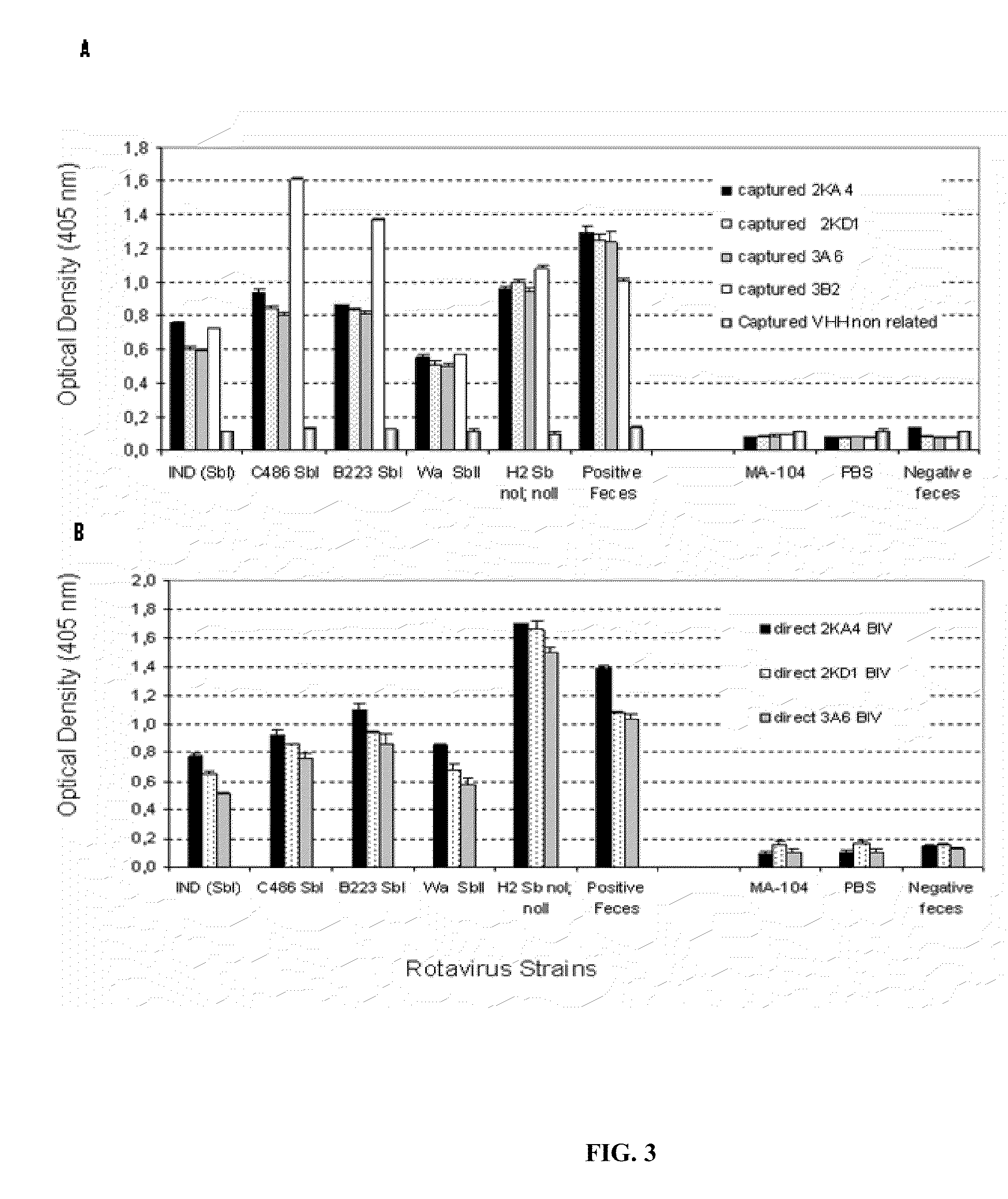
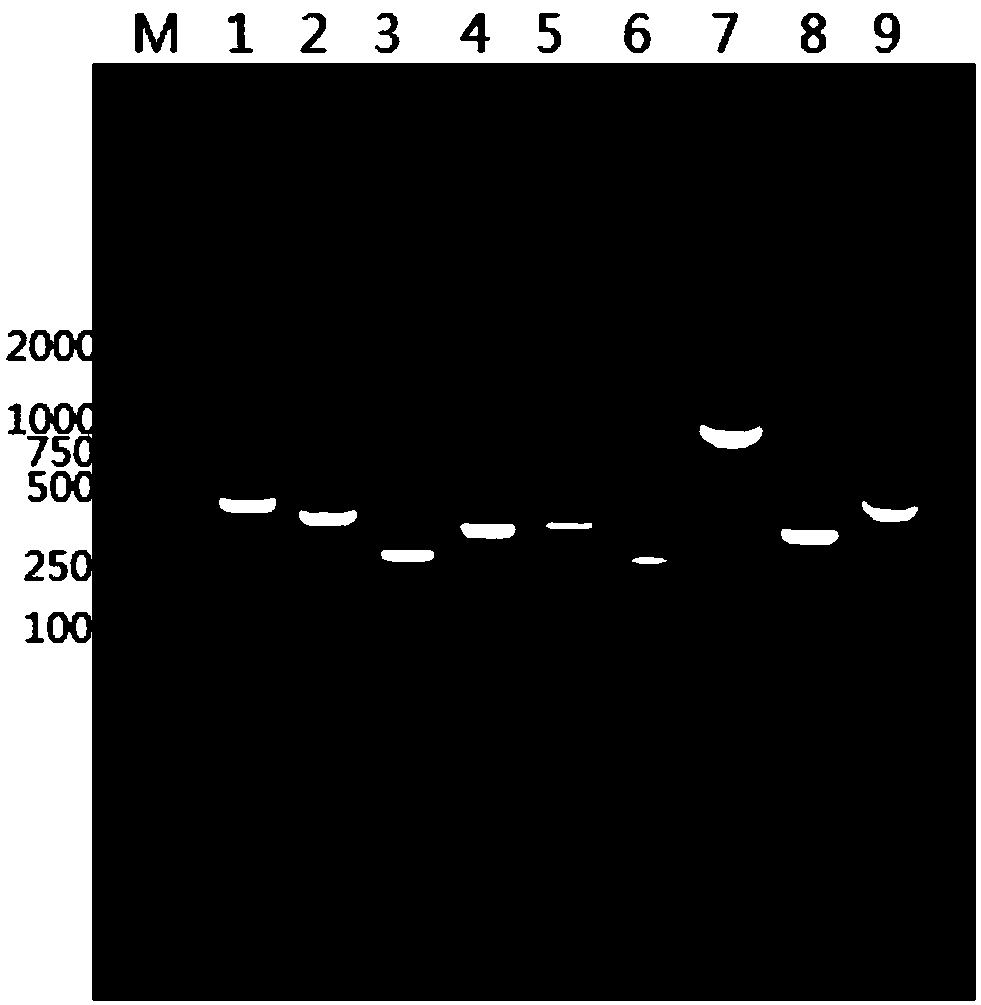
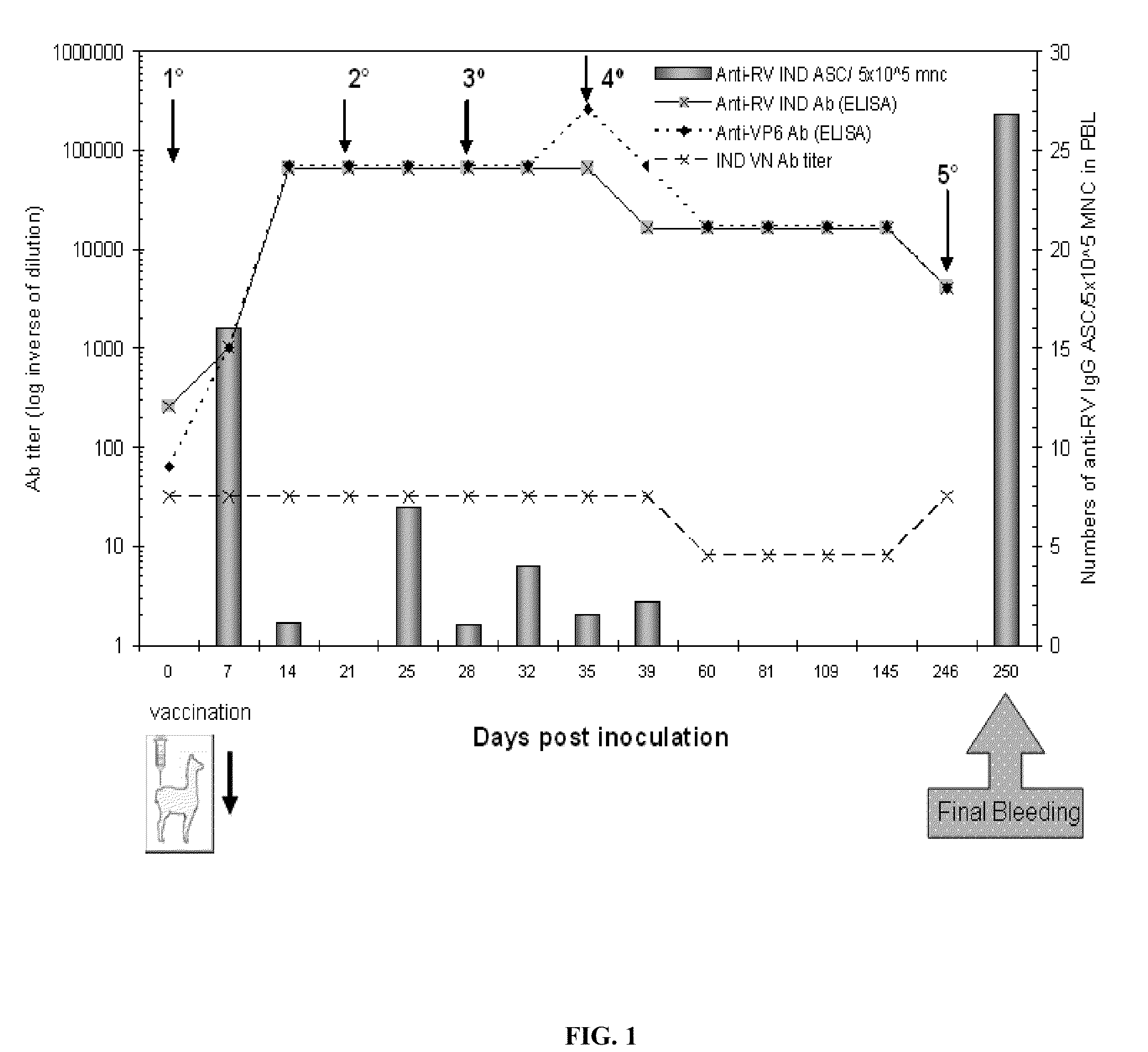
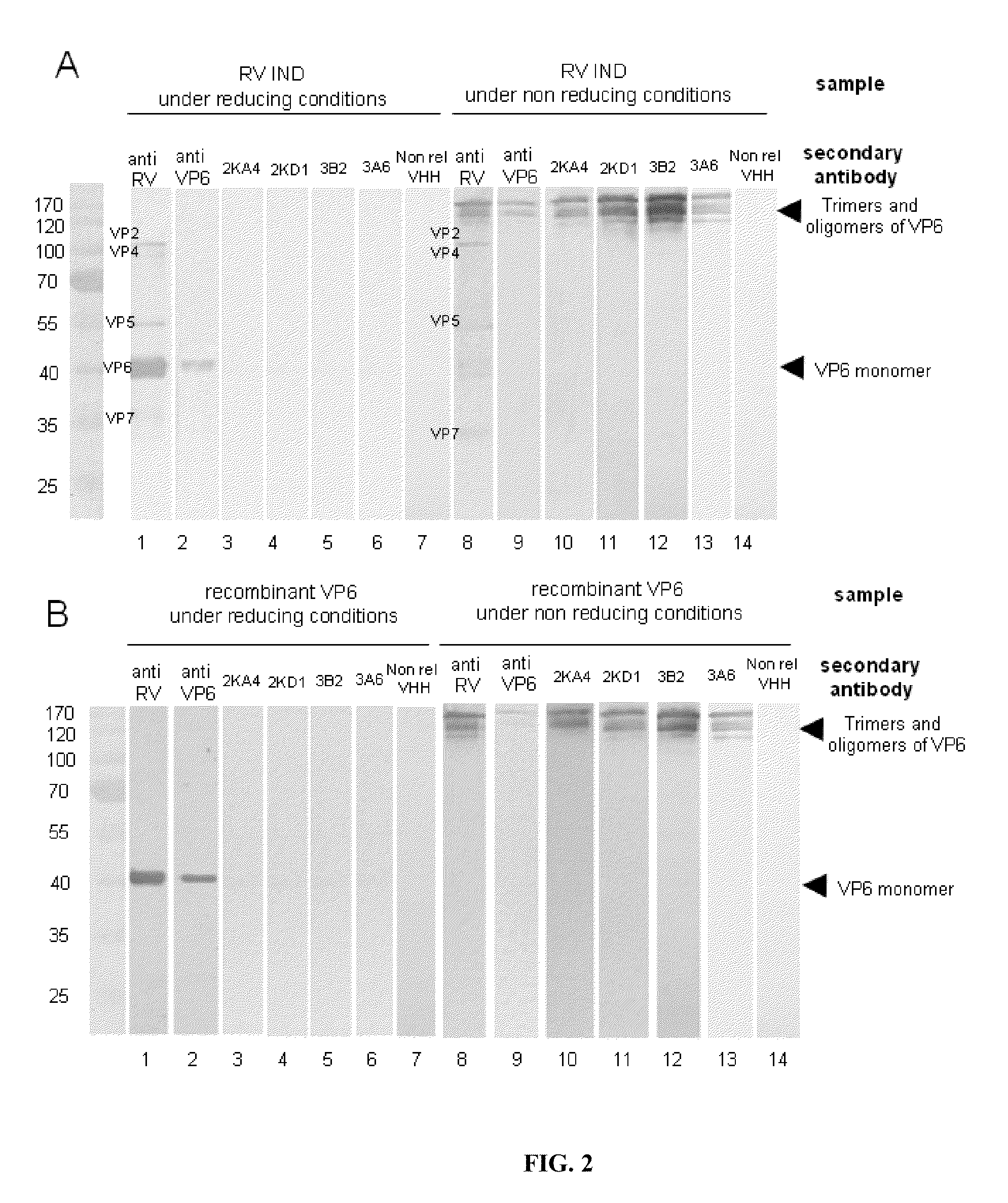
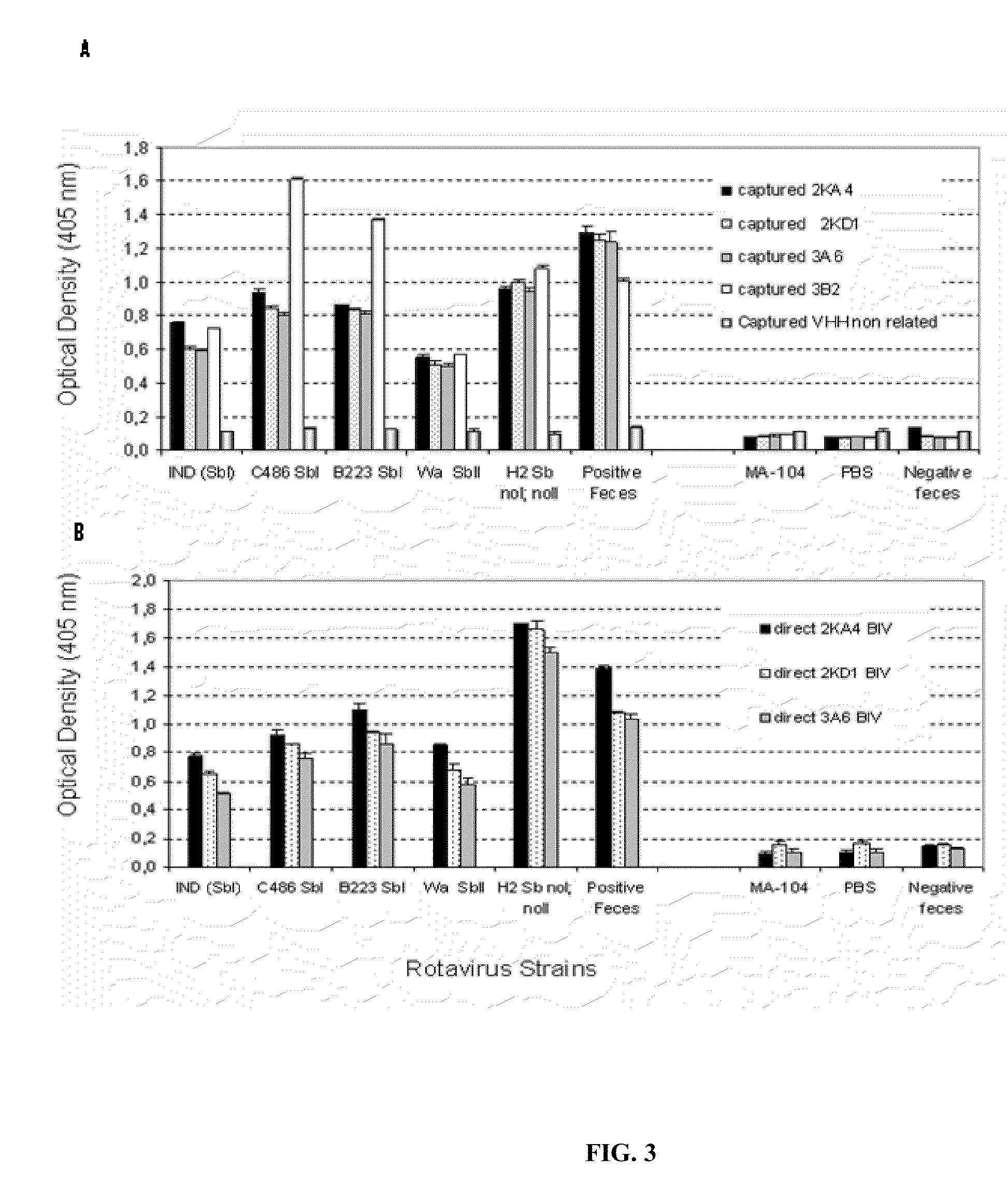
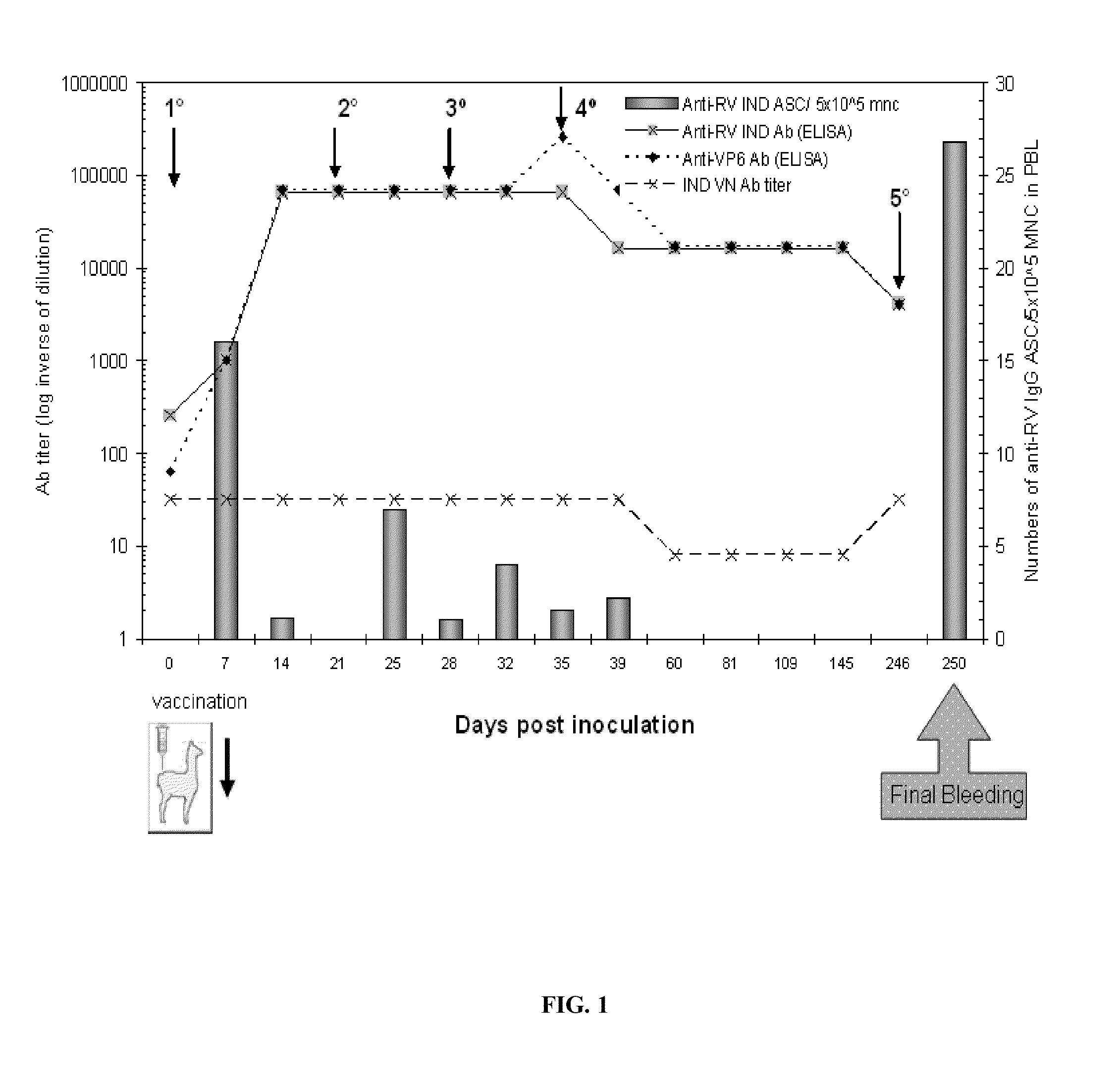
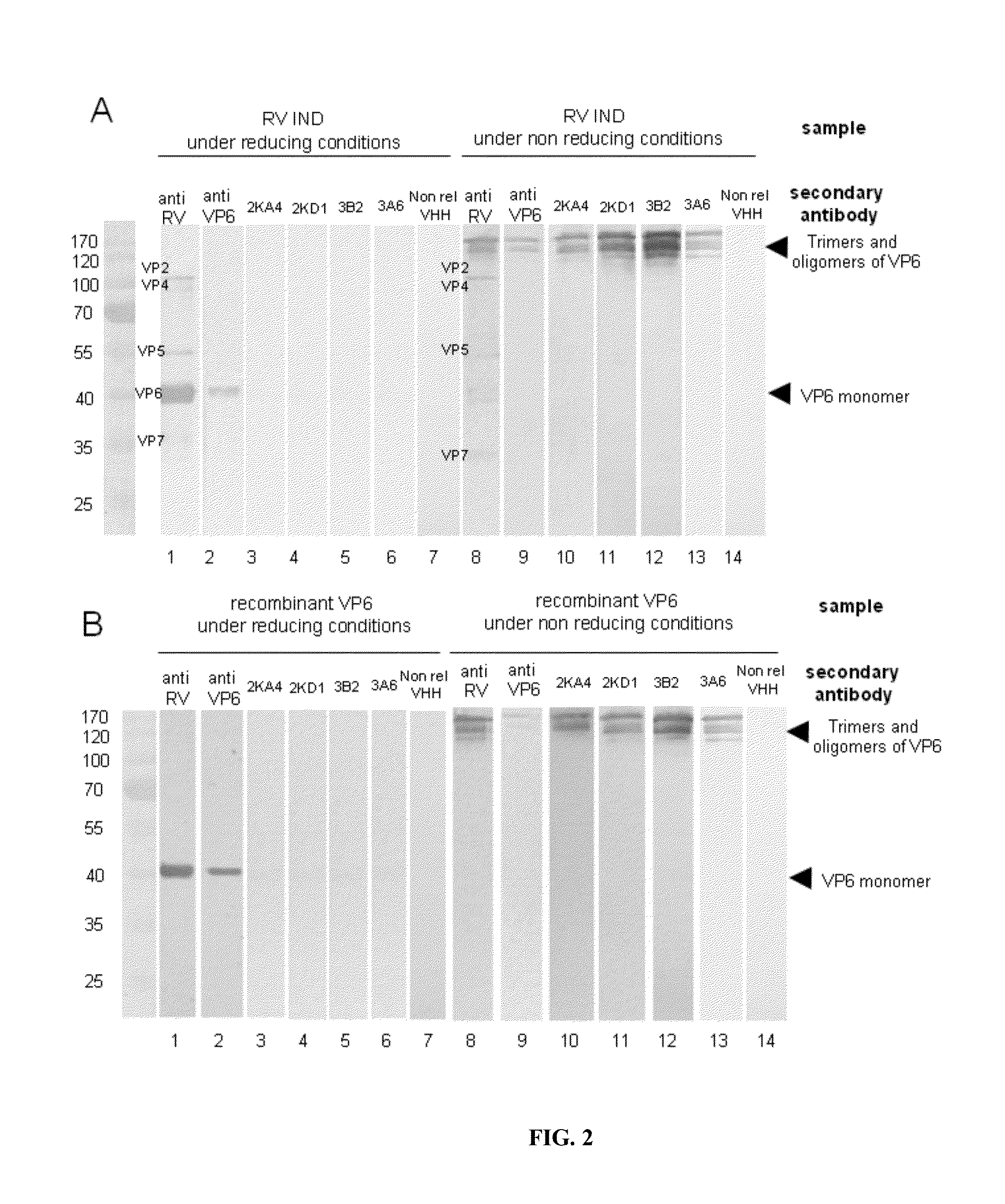
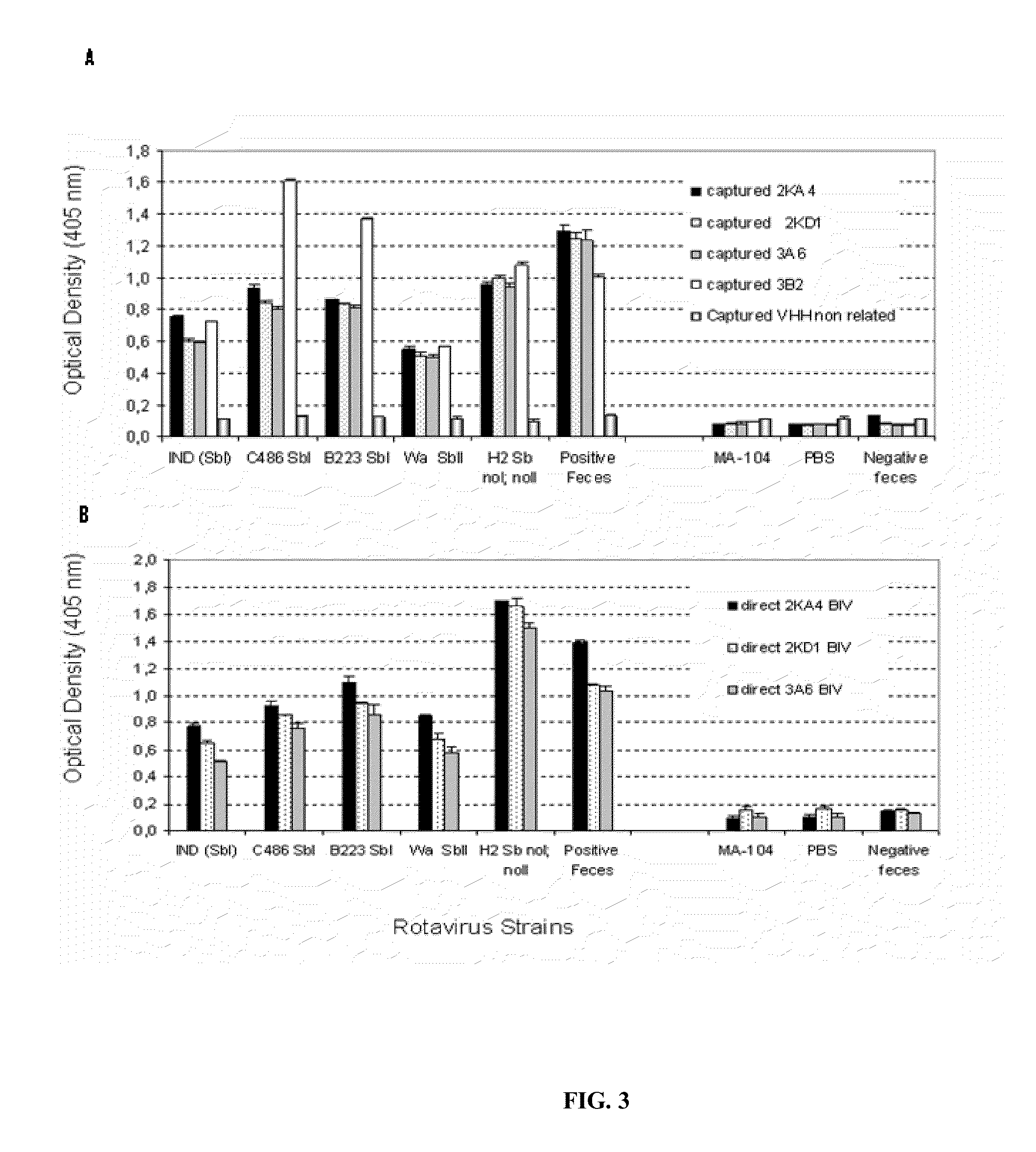
Monomeric vhh domain derived from Anti-vp6 camelid antibodies, dimeric domain, immunisation method, rotavirus detection method, composition, prevention and treatment methods for rotavirus infections
ActiveUS20100330068A1Peptide/protein ingredientsImmunoglobulins against virusesTGE VACCINEAmino acid
Monomeric VHH domain derived from anti-VP6 camelid antibodies, dimeric domain, immunisation method, rotavirus detection method, vaccine composition, prevention and treatment methods for rotavirus infections, wherein said domain may be any of the amino acid sequences shown in SEQ ID No. 1, SEQ ID No. 2, SEQ ID No. 3 or SEQ ID No. 4, and wherein said domains bind to protein VP6 of Group A rotavirus.
Owner:INST NACIONAL DE TECNOLOGIA AGROPECUARIA
Group A rotavirus chromatography test paper strip based on low-noise excitation fluorescent label
InactiveCN104502608AImprove signal-to-noise ratioHigh sensitivityBiological testingLow noiseQuantitative determination
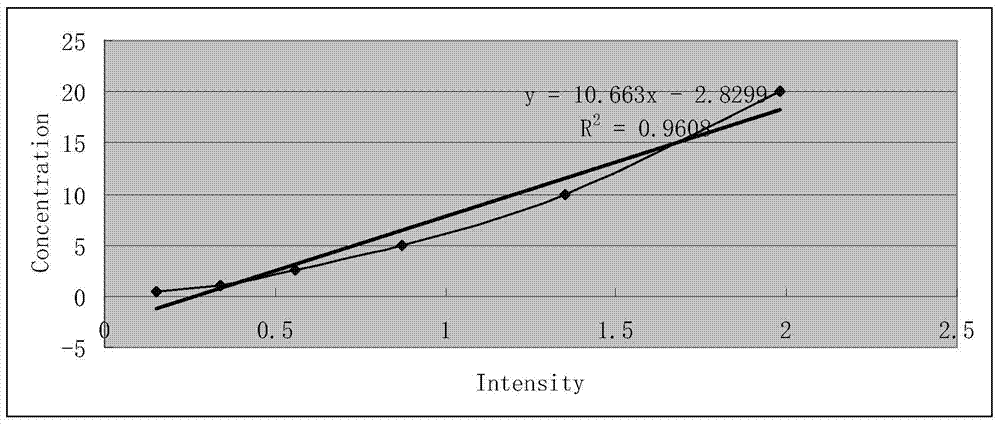
The invention belongs to the field of food detection, and particularly relates to a group A rotavirus chromatography test paper strip based on low-noise excitation fluorescent label as well as a preparation method and application of the chromatography test paper strip. According to the invention, quantum dots are adopted as the label, the immunochromatography technology is adopted, so that the chromatography test paper strip targeting to group A rotavirus is prepared, during detection, a special portable low-noise excitation fluorescent scanner is adopted for scanning a quality control line and a detection line, and qualitative and quantitative determination of a sample is realized through a fluorescence intensity detection value of the detection line. The test paper strip is used for immediate detection of group A rotavirus in food and a pathological sample, and has the advantages of rapidness, high sensitivity, qualitative, accurate and quantitative detection, and portability.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT +2
New method for rapid detection of G genotype of group A rotavirus by VP7 gene
InactiveCN102226221AReduce in quantityImprove efficiencyMicrobiological testing/measurementRotavirus RNANucleotide
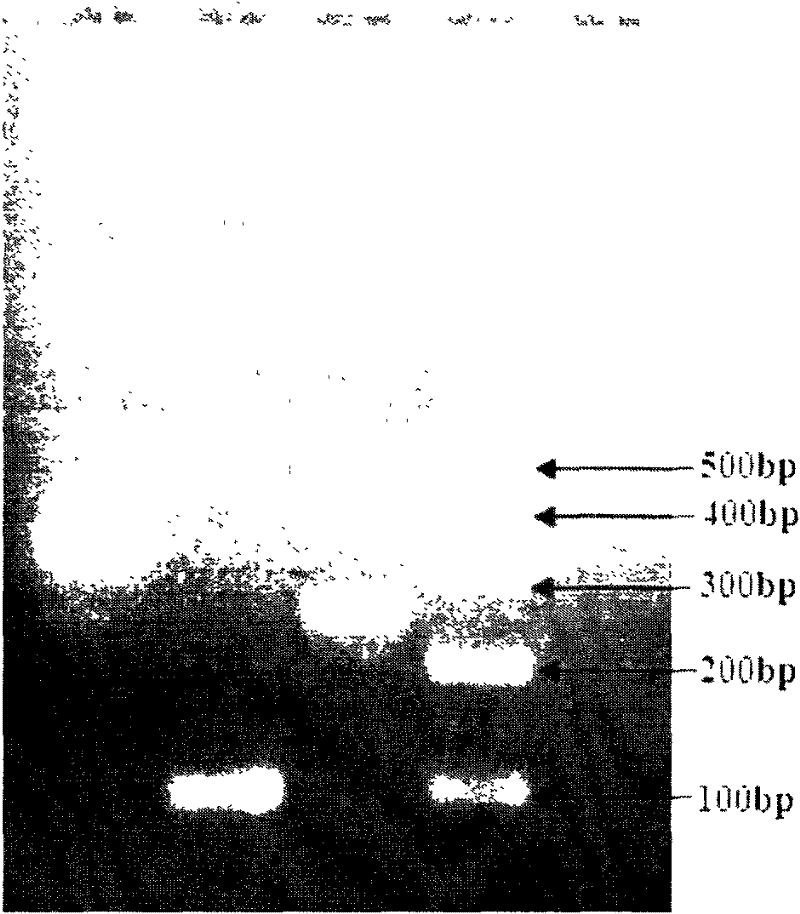
The invention provides a new method for rapid detection of G genotype of group A rotavirus by VP7 gene, comprising the steps of: 1) designing six nucleotide sequences capable of specifically amplifying different G genotypes of group A rotavirus as the primers for detecting the different G genotypes; 2) extracting virus nucleic acid from a sample and conducting one-time RT-PCR (reverse transcription-polymerase chain reaction) amplification with the six primers, and performing an electrophoresis detection to a product of PCR with 2% agarose gel, dyeing the product with ethidium bromide, and taking a picture by means of a gel imaging system; 3) judging the G genotype of group A rotavirus: when the size of the PCR product is respectively 332bp, 600bp, 98bp, 471bp and 248bp, the sample respectively contains G1, G2, G3, G4 and G9 rotaviruses, thus establishing a criteria for judging the G genotype of group A rotavirus.
Owner:PEOPLES HOSPITAL OF XINJIANG UYGUR AUTONOMOUS REGION
Traditional Chinese medicine for treating infant group A rotavirus infectious diarrhea
InactiveCN106620413AAvoid damageAchieve the purpose of healingDigestive systemPharmaceutical delivery mechanismTherapeutic effectTalc
The invention discloses a traditional Chinese medicine for treating infant group A rotavirus infectious diarrhea. The traditional Chinese medicine is characterized by comprising 10-15g of kudzu vine roots, 10-15g of scutellaria baicalensis, 10-15g of coptis chinensis, 5-10g of agastache rugosus, 5-10g of talc, 5-10g of radix saposhnikoviae, 5-10g of rhizoma atractylodis, 4-6g of liquorice, 8-12g of medicated leaven, 8-12g of roasted malt, 8-12g of hawthorn and 8-12g of poria cocos. The components are decocted twice till a decoction of 100mL is obtained, and 20-30mL of the decoction is kept being 37 DEG C for enema. The traditional Chinese medicine for treating infant group A rotavirus infectious diarrhea, which is disclosed by the invention, is capable of relieving stagnation and activating spleen, rhizoma atractylodis has the effects of drying humidity and tonifying spleens, exogenous pathogen can be removed, intestines can be cleared, humidity can be removed, and together with a retention enema administration way of the traditional Chinese medicine, the treatment effects of the traditional Chinese medicine can be brought into play, the phenomenon that spleens and stomachs are injured as the decoction enters the stomachs can be avoided, affected parts can be reached directly, and the purpose of treatment can be achieved.
Owner:SHAANXI YUHANG ELECTRONICS
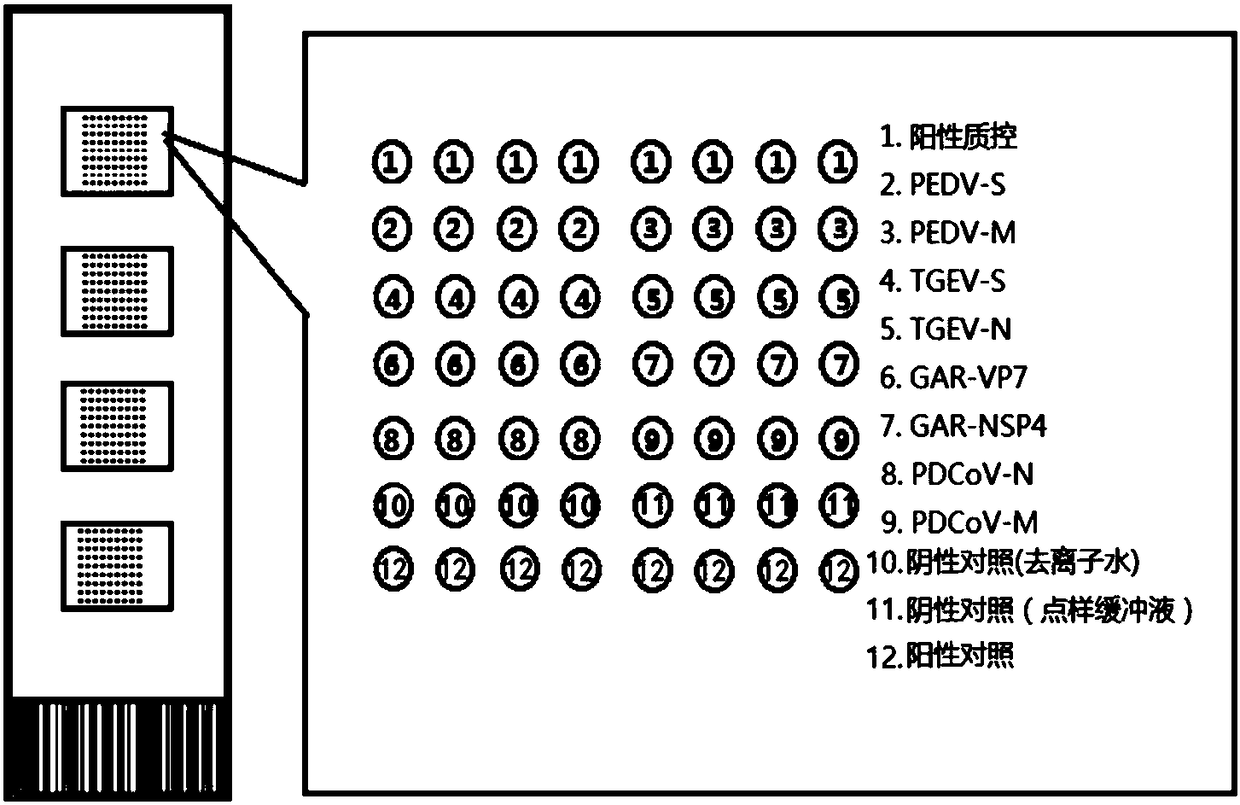
Oligonucleotide chip for synchronous detection of four porcine diarrhea viruses, and application of oligonucleotide chip
ActiveCN108531648ANucleotide librariesMicrobiological testing/measurementRotavirus RNAOligonucleotide chip
The invention provides an oligonucleotide chip for synchronous detection of four porcine diarrhea viruses. The oligonucleotide chip comprises a solid phase carrier and oligonucleotide probes fixed onthe solid phase carrier, wherein the oligonucleotide probes comprise a probe shown in SEQ ID No. 1-2, a probe shown in SEQ ID No. 3-4, a probe shown in SEQ ID No. 5-6, and a probe shown in SEQ ID No.7-8; the probes are respectively used for detecting porcine epidemic diarrhea virus (PEDV), porcine transmissible gastroenteritis virus (TGEV), porcine group A rotavirus (GAR) and porcine delta coronavirus (PDCoV). The invention also provides a kit and a method for the synchronous detection of the four porcine diarrhea viruses. The chip and the method can realize the high flux co-detection of thefour porcine diarrhea viruses, i.e., PEDV, TGEV, GAR and PDCoV, and can be applied to mass detection of clinical diseases, thus having a wide application prospect.
Owner:SICHUAN AGRI UNIV
Monomeric VHH domain derived from anti-VP6 camelid antibodies, dimeric domain, immunisation method, rotavirus detection method, composition, prevention and treatment methods for rotavirus infections
Owner:INST NACIONAL DE TECNOLOGIA AGROPECUARIA
Quadruple RT-PCR detection primers and kits for four porcine diarrhea viruses
ActiveCN110093461BStrong specificityRepeatableMicrobiological testing/measurementMicroorganism based processesViral testTransmissible gastroenteritis virus
The invention relates to quadruple RT-PCR detection primers and kits for porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus, porcine group A rotavirus and porcine deltacoronavirus, belonging to the technical field of molecular detection. The quadruple RT-PCR detection primers of the present invention include porcine epidemic diarrhea virus detection primers PEDV F and PEDV R; porcine transmissible gastroenteritis virus detection primers TGEV F and TGEV R; porcine group A rotavirus detection primers PoRV F and PoRV R; and porcine deltacoronavirus detection primers PDCoV‑F and PDCoV‑R. The primer of the invention has strong specificity, repeatability, high sensitivity and high clinical reliability.
Owner:ANHUI AGRICULTURAL UNIVERSITY
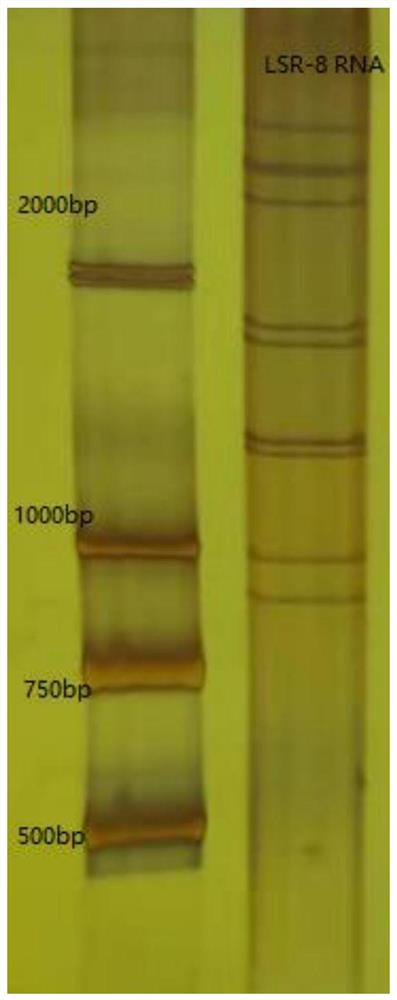
Rotavirus strain and application thereof as vaccine
The invention relates to the field of viruses, in particular to separation, culture and identification of a group A rotavirus G9P[8] strain and application of the group A rotavirus G9P[8] strain as a vaccine. Specifically, the invention relates to a group A rotavirus seed LSR-8 strain, and relates to a group A rotavirus seed LSR-8 strain; the total amino acid sequences of the group A rotavirus seed LSR-8 strain are shown as SEQ ID No.1 to SEQ ID No.11; and the total nucleic acid sequences of the group A rotavirus seed LSR-8 strain are shown as SEQ ID No.12 to SEQ ID No.22. The invention further relates to application of the LSR-8 strain in production of inactivated human rotavirus vaccines, human rotavirus attenuated live vaccines and combined vaccines; and the LSR-8 strain can be used for preventing, relieving or treating rotavirus infection and diseases caused by rotavirus infection, such as rotavirus gastroenteritis, diarrhea and the like.
Owner:山东威高利彤生物制品有限公司
Monomeric vhh domain derived from Anti-vp6 camelid antibodies, dimeric domain, immunisation method, rotavirus detection method, composition, prevention and treatment methods for rotavirus infections
Monomeric VHH domain derived from anti-VP6 camelid antibodies, dimeric domain, immunisation method, rotavirus detection method, vaccine composition, prevention and treatment methods for rotavirus infections, wherein said domain may be any of the amino acid sequences shown in SEQ ID No. 1, SEQ ID No. 2, SEQ ID No. 3 or SEQ ID No. 4, and wherein said domains bind to protein VP6 of Group A rotavirus.
Owner:INST NACIONAL DE TECNOLOGIA AGROPECUARIA
Oral hexavalent reassortment live rotavirus vaccine
PendingCN113730564APrevent diarrheaViral antigen ingredientsInorganic non-active ingredientsInfantile diarrheaSerotype
The invention relates to an oral hexavalent reassortment live rotavirus vaccine. The vaccine contains six main epidemic strain serotypes G1, G2, G3, G4, G8 and G9, covers 99.6% of G serotype virus of group A rotavirus, and is expected to have a good prevention and protection effect on rotavirus diarrhea. The invention aims to provide an oral inoculated live vaccine consisting of serotypes of hexavalent rotavirus for preventing infantile diarrhea caused by rotavirus, and the vaccine comprises the following components: (1) G1, G2, G3, G4, G8 and G9 serotype live vaccines with a stock solution titer of each serotype being 1-5 * 10 < 6 > FFU / ml; and (2) a protective agent: 0.5-2 g / L of citric acid, 50-150 g / L of sodium citrate, 250-450 g / L of sucrose, 5-10 mM of zinc chloride and 10-20 mM of calcium chloride.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Quadruple fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for detecting porcine epidemic diarrhea virus and porcine rotavirus and application of quadruple fluorescent quantitative PCR detection kit
ActiveCN114807437ALow costProof of feasibilityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic diarrheaEpidemiologic study
The invention discloses a quadruple fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for detecting porcine epidemic diarrhea virus and porcine rotavirus and application of the quadruple fluorescent quantitative PCR detection kit. The kit comprises a hot start Taq DNA polymerase, enzyme-free water, a PCR reaction solution and a probe method fluorescent quantitative PCR matched Buffer, the four pairs of specific primers, the corresponding TaqMan probes and the reference substances are used for detecting the G1 genotype porcine epidemic diarrhea virus, the G2 genotype porcine epidemic diarrhea virus, the porcine group A rotavirus and the porcine group C rotavirus. The kit provided by the invention realizes simultaneous detection of four viruses capable of causing porcine diarrhea in one PCR reaction tube, has excellent specificity and repeatability, and can be directly applied to conventional laboratory diagnosis of porcine clinical diarrhea samples; and a rapid and accurate detection method is provided for epidemiological research of the porcine epidemic diarrhea virus and the porcine rotavirus.
Owner:NANJING AGRICULTURAL UNIVERSITY
Composition, kit and method for detecting group A rotavirus
ActiveCN102260752BAccelerated deathBiohazard ReductionMicrobiological testing/measurementFluorescence/phosphorescenceMicrobiologyGenome
The invention discloses a composition for detecting group A rotavirus, belonging to the technical field of biology, comprising the following primers: 5'-GGCTTTAAAAGAGAGAATTTCC-3' and 5'-AAYTGYGGTATATTCAATACCATACA-3', also comprising the following probes: 5'-fluorescent reporter dye AARGAGCTAWCCGYTAGCCAGACGG-fluorescence quencher-3'. The invention further discloses a kit containing the primers andprobes and a method for detecting group A rotavirus. When using the composition, the kit and the / or method to detect group A rotavirus, a genome equivalent / reaction system with the range of 10<8>-10<1> copies is detected, the detection is rapid and simple, and the whole detection process can be completed within 3 hours with high specificity and high accuracy.
Owner:成都新基因格生物科技有限公司
Multiplex pcr rapid diagnostic kit for five kinds of porcine diarrhea virus and its application
ActiveCN107419034BReduce mortalityReduce economic lossMicrobiological testing/measurementAgainst vector-borne diseasesSpecific detectionPorcine Circoviruses
The invention discloses a multi-PCR (polymerase chain reaction) rapid diagnostic kit for five porcine diarrhea viruses. The multi-PCR rapid diagnostic kit comprises five pairs of PCR primers, PCR reaction liquid, a PCR standard substance, a positive control substance and a negative control substance. The invention further discloses application of the kit in simultaneous specific detection of infection of the five porcine diarrhea viruses. The five porcine diarrhea viruses include TGEV (transmissible gastroenteritis virus), GAR (porcine group A rotaviruses), GCR (porcine group C rotaviruses), PEDV (porcine epidemic diarrhea virus) and PCV2 (porcine circovirus 2).
Owner:杭州洪桥中科基因技术有限公司 +1
Bovine colostrum product resisting infantile diarrhea
The invention relates to a bovine colostrum product resisting infantile diarrhea, in particular to a bovine colostrum product resisting rotavirus. The bovine colostrum product is achieved by the steps as follows: preparation of group A rotavirus (ARV), immunization of group A rotavirus (ARV) antigen, collection of immune milk, and preparation of milk product by the immune milk. The prepared bovine colostrum product resisting infantile diarrhea in the invention not only has a very good effect on preventing infantile diarrhea but also has the advantages of being excellent in specificity, small in side effects, simple and convenient in preparation method, easy for industrialization, not apt to generate drug-resistant strains, and the like.
Owner:江西英雄乳业股份有限公司
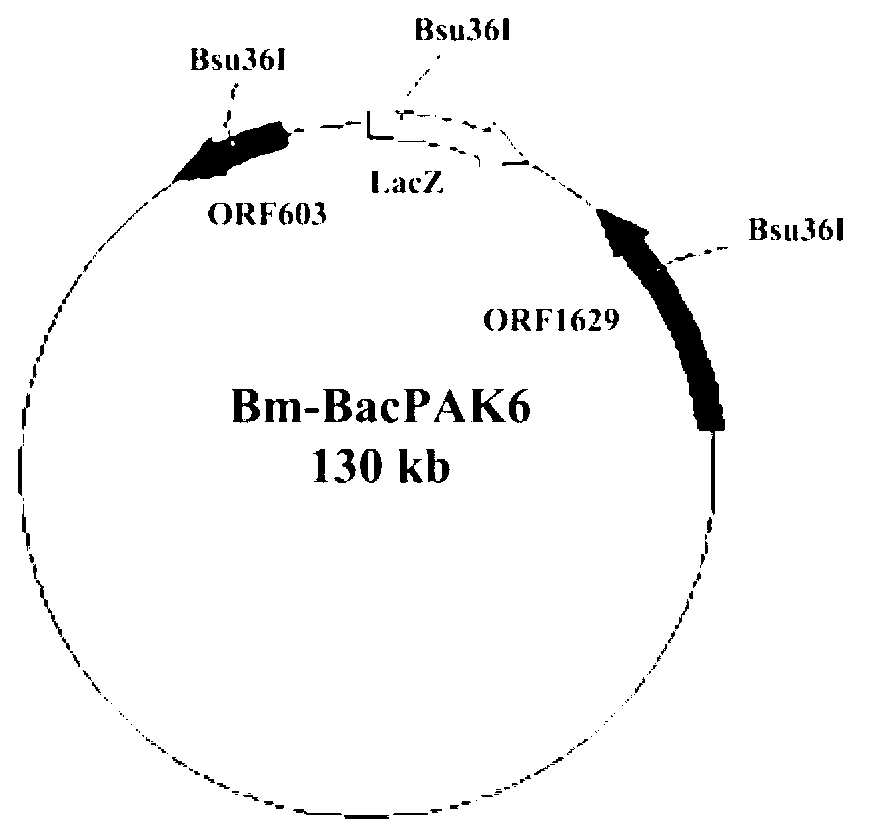
Method of expressing rotavirus virus protein 6 (VP6) protein by using silkworm bioreactor
InactiveCN102978240ATake advantage ofEasy to operateFermentationVector-based foreign material introductionVp6 geneVirus Protein
The invention discloses an acquiring method of a recombinant virus Bm nucleopolyhedrovirus (NPV)-rotavirus virus protein 6 (RVVP6). The method comprises the following steps: firstly, choosing group A rotavirus VP6 genes; secondly, constructing a recombinant transfer vector plasmid pBacPAK8-RVVP6; thirdly, introducing the constructed recombinant transfer vector plasmid pBacPAK8-RVVP6 and linearized Bm-BacPAK6 virus DNA into silkworm cells and co-transfecting the silkworm cells in a liposome mediated mode to acquire recombinant virus BmNPV-RVVP6. According to the method of expressing rotavirus VP6 protein by using a silkworm bioreactor, the technology includes that a silkworm BmN, larvae at the age of five and silkworm pupas are transfected by the BmNPV-RVVP6, recombinant protein VP6 is enabled to be expressed in the silkworm. According to the method of expressing rotavirus VP6 protein by using a silkworm bioreactor, raw materials are easy to acquire, and the method is low in production cost and non-toxic, and provides novel methods for studying and producing novel rotavirus vaccines.
Owner:ZHEJIANG SCI-TECH UNIV
An enzymatic visualized oligonucleotide chip for simultaneous detection of four porcine diarrhea viruses and its application
ActiveCN108531649BEasy to prepareQuick checkNucleotide librariesMicrobiological testing/measurementOligonucleotide chipNucleotide
The invention provides an enzymatic visualized oligonucleotide chip for synchronously detecting four porcine diarrhea viruses, which comprises a solid phase carrier and oligonucleotide probes immobilized on the solid phase carrier, which are used to detect porcine epidemics respectively Diarrhea virus, porcine transmissible gastroenteritis virus, porcine group A rotavirus, and porcine deltacoronavirus. The present invention also provides a method for preparing the above-mentioned chip. The invention also provides a kit and a detection method for synchronous detection of four porcine diarrhea viruses. The invention combines enzymatic reaction and gene chip technology, can quickly detect and differentially diagnose four kinds of porcine diarrhea virus diseases, has good specificity, high accuracy, and the detection results are visible to the naked eye, and provides a method for the prevention and control of porcine viral diarrhea A new diagnostic technology; moreover, the preparation method of the chip of the present invention is simple and low in cost, and can be applied to a large number of detections of clinical epidemic diseases, providing technical support for ensuring the healthy development of my country's pig industry.
Owner:SICHUAN AGRI UNIV
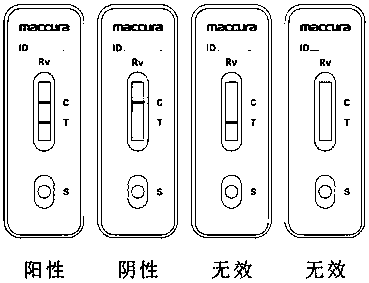
Group a rotavirus test strips
ActiveCN109613239BImprove anti-interference abilityHigh detection sensitivityBiological material analysisMicrobiologyImmunochromatographic test
The invention relates to a colloidal gold immunochromatographic test paper for detecting group A rotavirus. The colloidal gold immunochromatographic test paper includes a chromatographic membrane, and the chromatographic membrane is provided with a detection line and a quality control line; wherein , the colloidal gold is coated with a first anti-group A rotavirus monoclonal antibody, and the detection line is coated with a second anti-group A rotavirus monoclonal antibody. The invention also relates to corresponding reagents. Improve anti-interference ability and sensitivity when detecting group A rotavirus.
Owner:SICHUAN MACCURA BIOTECH CO LTD
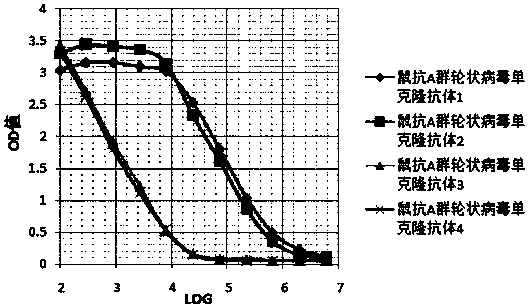
Hybridoma Cells Secreting Murine Anti-group A Rotavirus Monoclonal Antibody and Its Secreted Monoclonal Antibody
ActiveCN107287167BBiological material analysisImmunoglobulins against virusesAntiendomysial antibodiesHybridoma cell
The invention relates to a hybridoma cell capable of secreting a rat anti-A group rotavirus monoclonal antibody and a secreted monoclonal antibody. The invention also relates to an application of the monoclonal antibody for preparing a detection test strip used for detecting A group rotavirus in a sample A and the detection test strip containing the monoclonal antibody. The monoclonal antibody according to the invention is high in specificity, sensitivity and stability and can be used for quickly and accurately detecting the A group rotavirus in the sample.
Owner:SICHUAN ANKERUI NEW MATERIAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com