Group-A rotavirus rapid real-time fluorescent RT-PCR (reverse transcription-polymerase chain reaction) detection kit
A group A rotavirus, RT-PCR technology, applied in the direction of DNA / RNA fragments, recombinant DNA technology, microbial measurement / inspection, etc., can solve the problems of acute onset, lack of sensitivity, difficult to promote, etc., to improve reliability and accuracy, fast detection speed, avoiding false negatives and false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
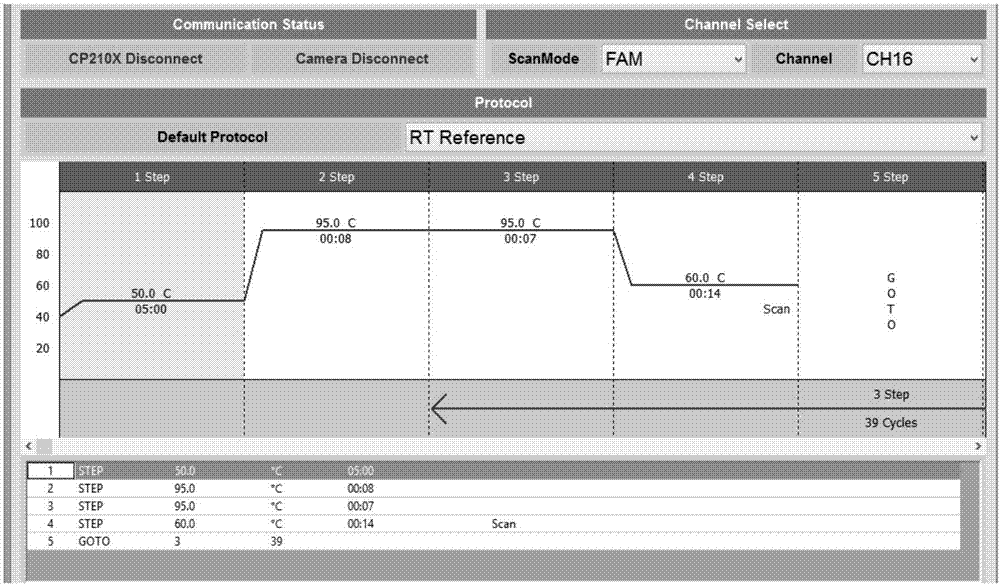
[0053] Example 1: Preparation and optimization of the rapid real-time fluorescent RT-PCR detection kit for group A rotavirus according to the present invention
[0054] 1. Design of primers and probes: through sequence comparison and analysis of the nucleic acid sequences of the reported group A rotaviruses, a segment with no secondary structure and a high degree of conservation was selected. According to the basic principles of primer and probe design, the software And artificially design multiple pairs of primers and probes.
[0055] 2. Selection of clinical samples: According to relevant domestic and foreign literature reports, select stool samples.
[0056] 3. Establishment and optimization of the reaction system
[0057] Screening of primer probes: Use the multiple sets of primer probes designed in 1 above to detect the RNA of the above-mentioned positive quality control products and negative quality control products. Primer Probe Assemblies. (The forward primer sequen...
Embodiment 2
[0065] Embodiment 2: use the detection method of group A rotavirus rapid real-time fluorescent RT-PCR detection kit of the present invention
[0066] 1. Specimen collection, transportation and storage
[0067] Take 500-1000μl of the sample to be tested and the watery diarrhea, put it into a sterile 1.5ml centrifuge tube, and send it directly for testing or store it at -20°C, and the storage period should not exceed six months. Ship sealed in dry ice to avoid freeze-thaw.
[0068] 2. Nucleic acid extraction
[0069] Take 200ul water sample diarrhea and add it to a 1.5ml centrifuge tube, vortex fully, centrifuge at 5000rpm for 30s, take 50ul supernatant and add 5ul lysate, which consists of 0.5% (w / v) sodium lauroyl sarcosinate, 200mmol / L dithiothreitol, TE (pH7.4), incubate at 95°C for 2min, centrifuge at 12,000rpm for 3min, collect the supernatant, which can be directly used for PCR detection or temporarily stored at -20°C. If stored for more than one month, it can be store...
Embodiment 3
[0084] Embodiment 3: the use of the quality control product in the rapid real-time fluorescent RT-PCR detection kit of group A rotavirus of the present invention
[0085] The quality control substances in the rotavirus rapid PCR kit include positive quality control substances and negative quality control substances, which are used for quality control in clinical trials. The operation method is the same as that of the samples to be tested, see Example 2.
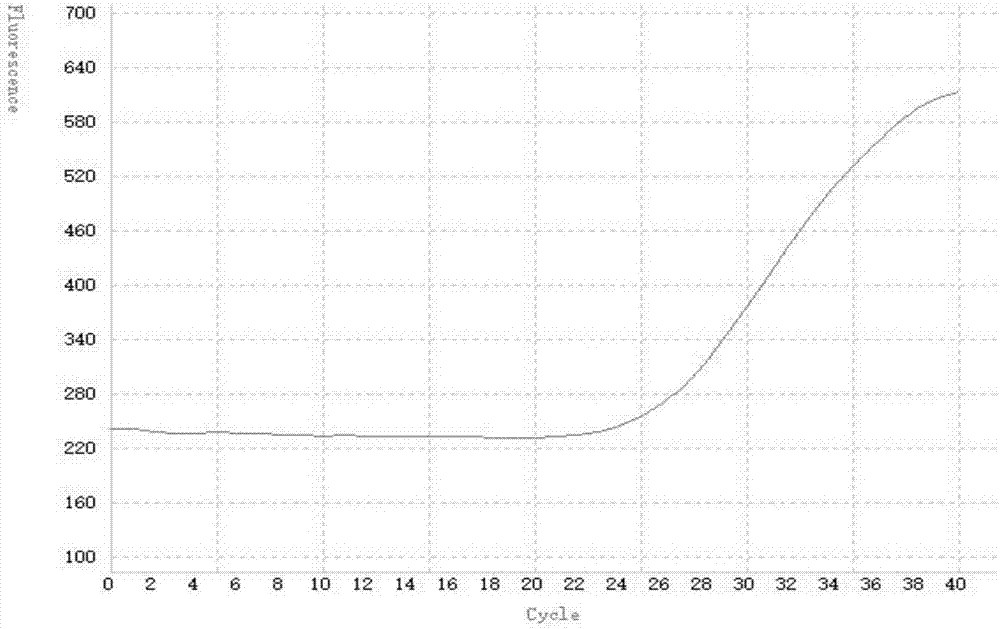
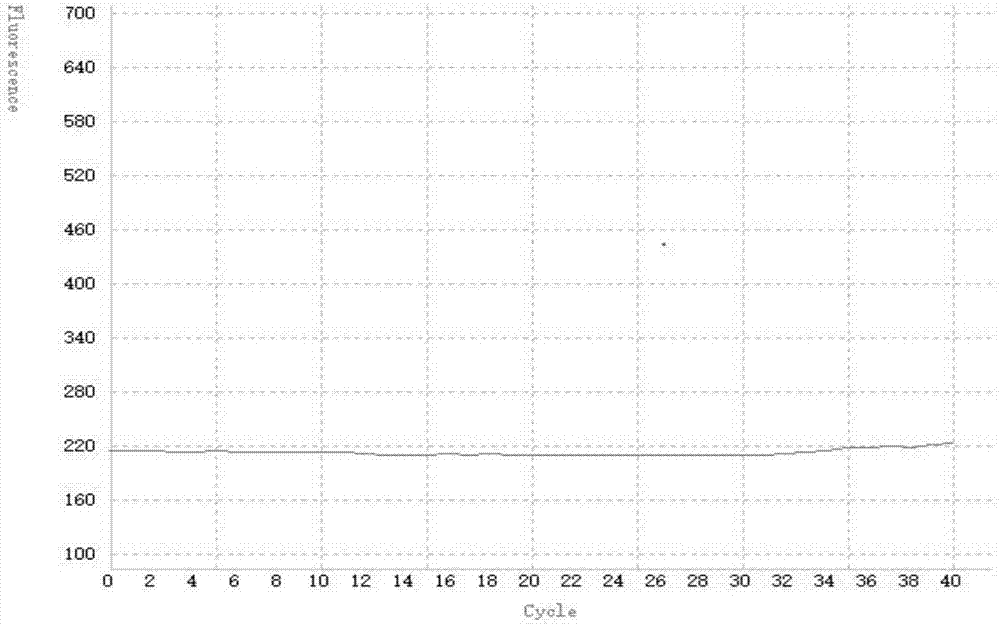
[0086] The result is as follows:
[0087] The amplification curve of the positive quality control product is S-shaped and the Ct value is ≤33, see the attached figure 2 , the amplification curve shown in the figure is an S-shaped curve, indicating that the detection system effectively amplifies the group A rotavirus nucleic acid.
[0088] The amplification curve of the negative quality control product is not S-shaped, see the attached image 3 , the graph shows that the amplification curve is a relatively straight broken l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com