Kit for detecting FLT3-ITD (Fms-like tyrosine kinase 3-internal tandem duplication) gene mutation by using fluorescence PCR (Polymerase Chain Reaction) capillary electrophoresis
A FLT3-ITD, kit technology, applied in the direction of microbial determination/examination, biochemical equipment and methods, etc., can solve problems such as poor prognosis, achieve improved reliability and accuracy, high sensitivity, avoid false negatives and The effect of false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: detection kit and its use
[0035] 1. Prepare a kit comprising the following components: ITD PCR reaction solution A (containing SEQ ID NO: 1 and SEQ ID NO:: 2) (500 μl / tube), ITD PCR reaction solution B (75 μl / tube), ITD Positive quality control (50μl / tube), negative quality control (50μl / tube).
[0036] 2. Specimen collection, transportation and storage:
[0037] (1) Specimen collection: The specimen is peripheral blood or bone marrow. Take 3-5ml of venous blood or bone marrow from the subject, inject it into a glass tube containing EDTA (disodium ethylenediaminetetraacetic acid) or sodium citrate anticoagulant, and immediately invert the glass tube gently for 5-10 times to mix the anticoagulant. The coagulant is thoroughly mixed with the venous blood.
[0038] (2) Storage: It can be detected immediately, stored at 4°C for one week, and stored at -20°C for up to one year.
[0039] (3) Transportation: Specimens should be transported using 0°C curling....
Embodiment 2
[0059] Example 2: Detection of FLT3-ITD in AML patients by QF-PCR amplification of the FLT3 locus
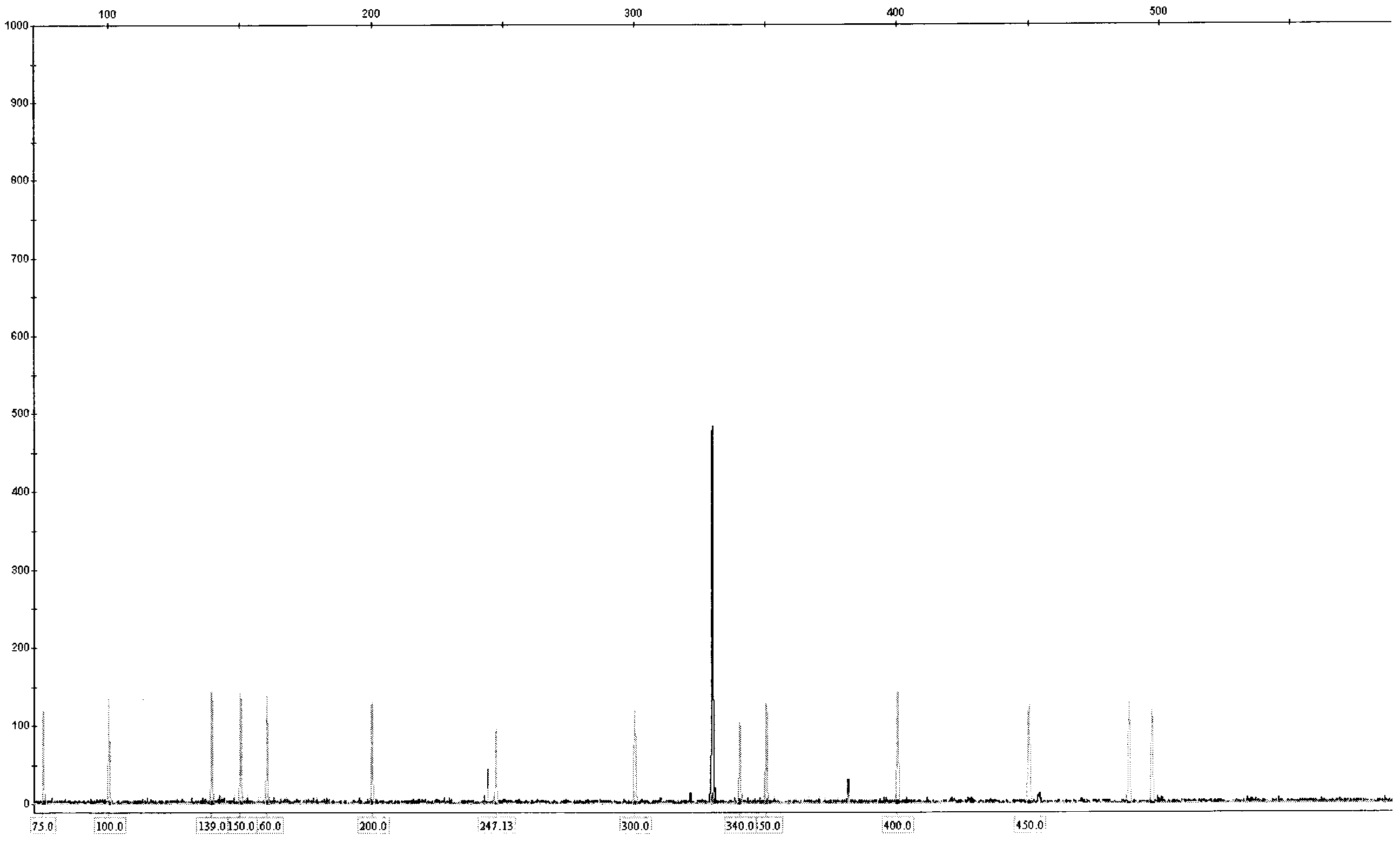
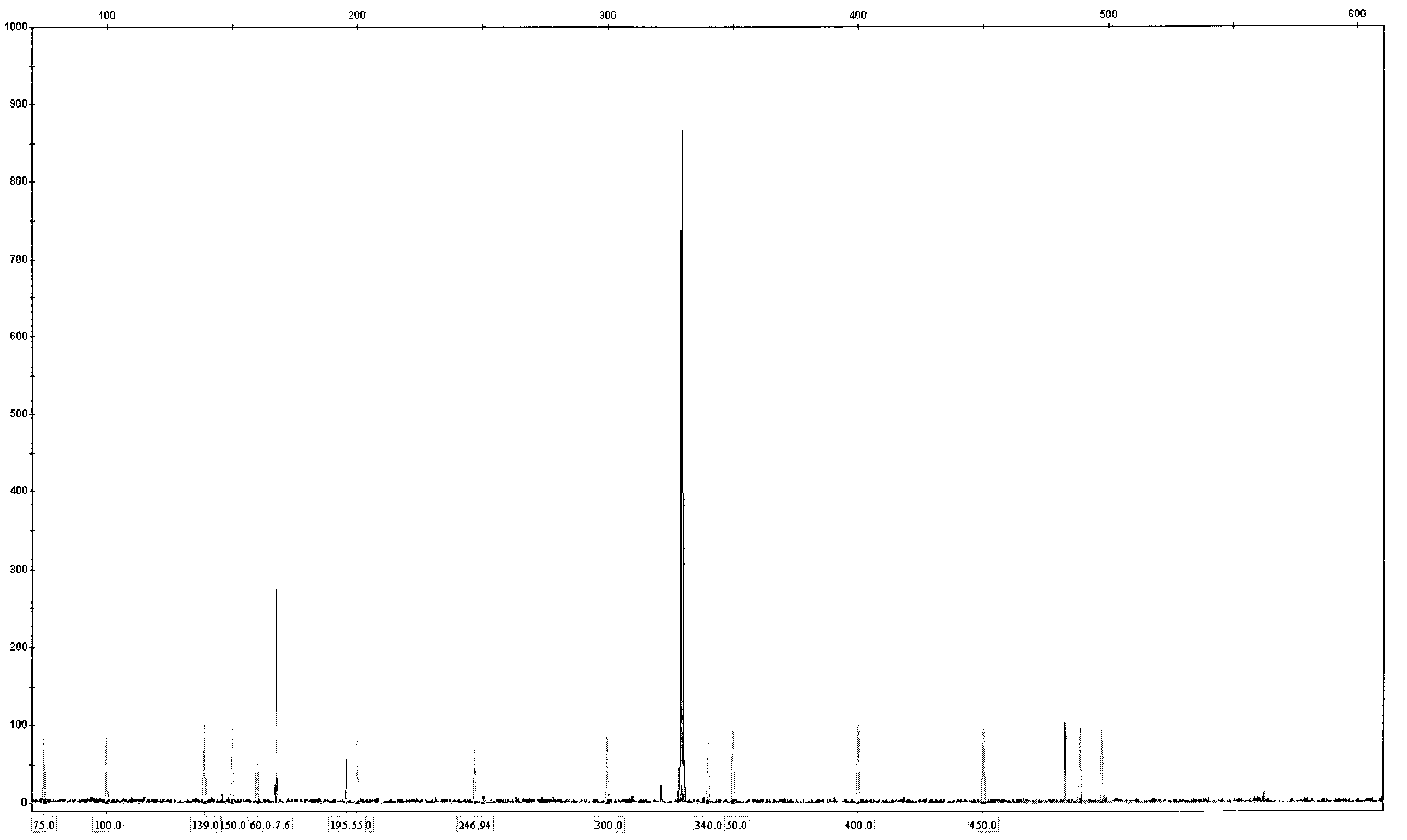
[0060] Blood or bone marrow samples from AML patients were subjected to DNA extraction and purification according to the standard procedure of Qiagen DNA extraction kit. Use the ITD PCR reaction solution A and ITD PCR reaction solution B provided in the kit to carry out the amplification reaction and load the sample for analysis according to the kit detection protocol. The analysis spectrum of the sample loading results is as follows: image 3 , Figure 4 . Wild-type and ITD loci on the FLT3 gene as shown. The ITD mutation in the FLT3 gene will produce an amplified band larger than the wild-type fragment. Thus, FLT3-ITD can be rapidly detected.
Embodiment 3
[0061] Example 3: Detection of FLT3-ITD in MDS patients by QF-PCR amplification of the FLT3 locus
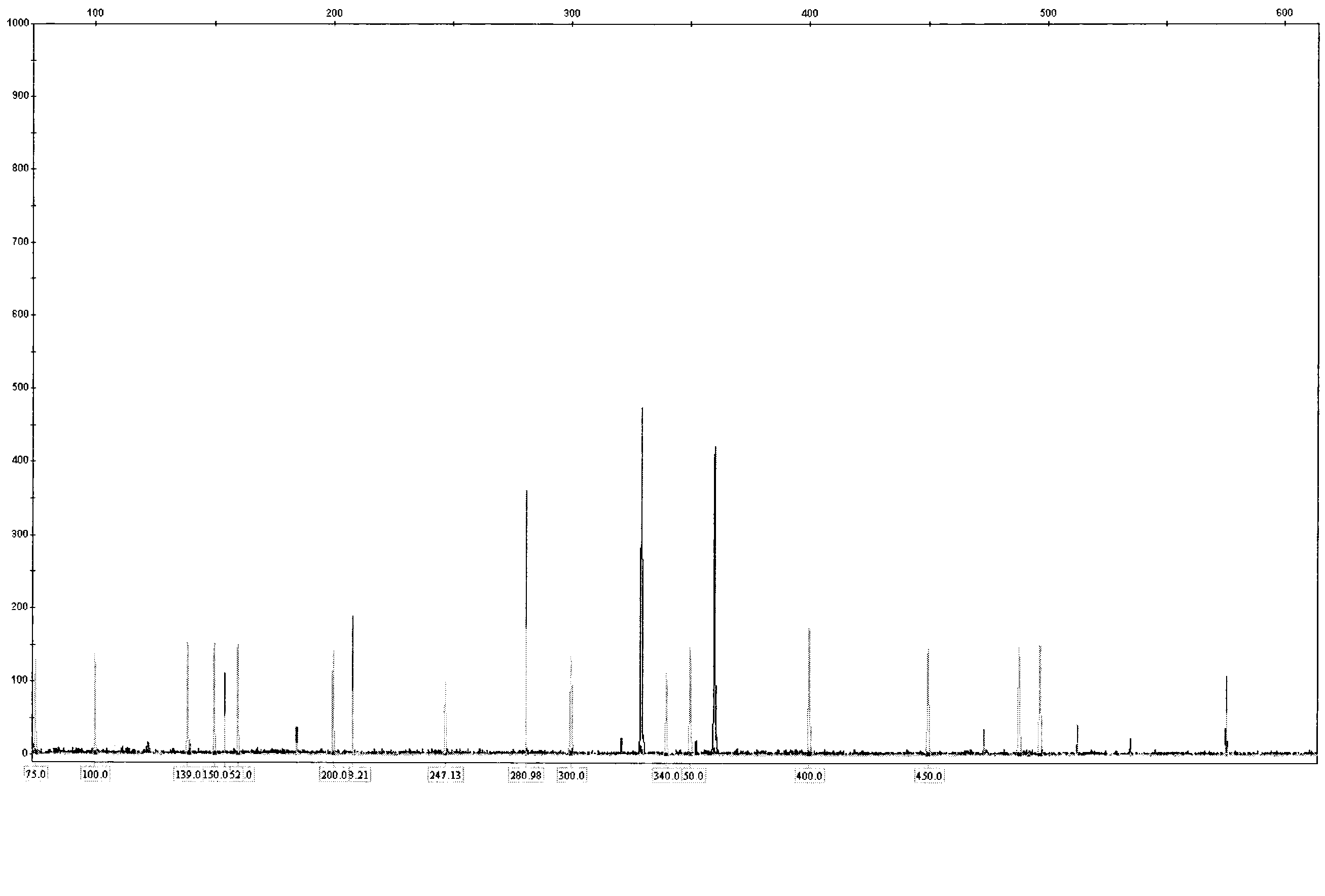
[0062] Blood or bone marrow samples from MDS patients were subjected to DNA extraction and purification according to the standard procedure of Qiagen DNA extraction kit. Use the ITD PCR reaction solution A and ITD PCR reaction solution B provided in the kit to carry out the amplification reaction and load the sample for analysis according to the kit detection protocol. The analysis spectrum of the sample loading results is as follows: Figure 5 . Wild-type and ITD loci on the FLT3 gene as shown. The ITD mutation in the FLT3 gene will produce an amplified band larger than the wild-type fragment. Thus, FLT3-ITD can be rapidly detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com