Yolk antirotavirus antibody preparation, method for preparing same and use thereof
A rotavirus and preparation technology, applied in the direction of antibody, drug combination, digestive system, etc., can solve the problem of weakening, achieve high neutralizing activity, simple and practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Isolation of Rotavirus
[0036] Isolation of human pathogenic rotavirus
[0037] Take 10ml of fecal juice from the child with diarrhea, dilute it 1:5 with 0.15M PBS with pH=7.6, put it into a sterile centrifuge tube, shake it fully for 30 minutes, add trypsin with a final concentration of 10μg / ml, and dilute the resulting solution in Incubate at 37°C for 20min. Aspirate the suspension and centrifuge at a high speed of 10000rpm / min. After the supernatant was filtered through a 0.22um filter, the filtrate was serially diluted 10 times with a virus proliferation culture note (MEM medium added with 2 μg / ml trypsin solution), and the resulting solution was used for the next step of amplification and Identification.
[0038] After the Vero cells were cultured to a single layer in a 6-well culture plate, the culture medium was discarded, and 1 ml of each virus isolation material mentioned above was added to each culture well. After incubating at 37°C for 60 min,...
Embodiment 2
[0040] Embodiment 2: In vitro culture and amplification of rotavirus
[0041] The isolated rotavirus culture is mixed with the rotavirus strain which is the standard human type Wa virus strain, and then co-cultivated and amplified in vitro.
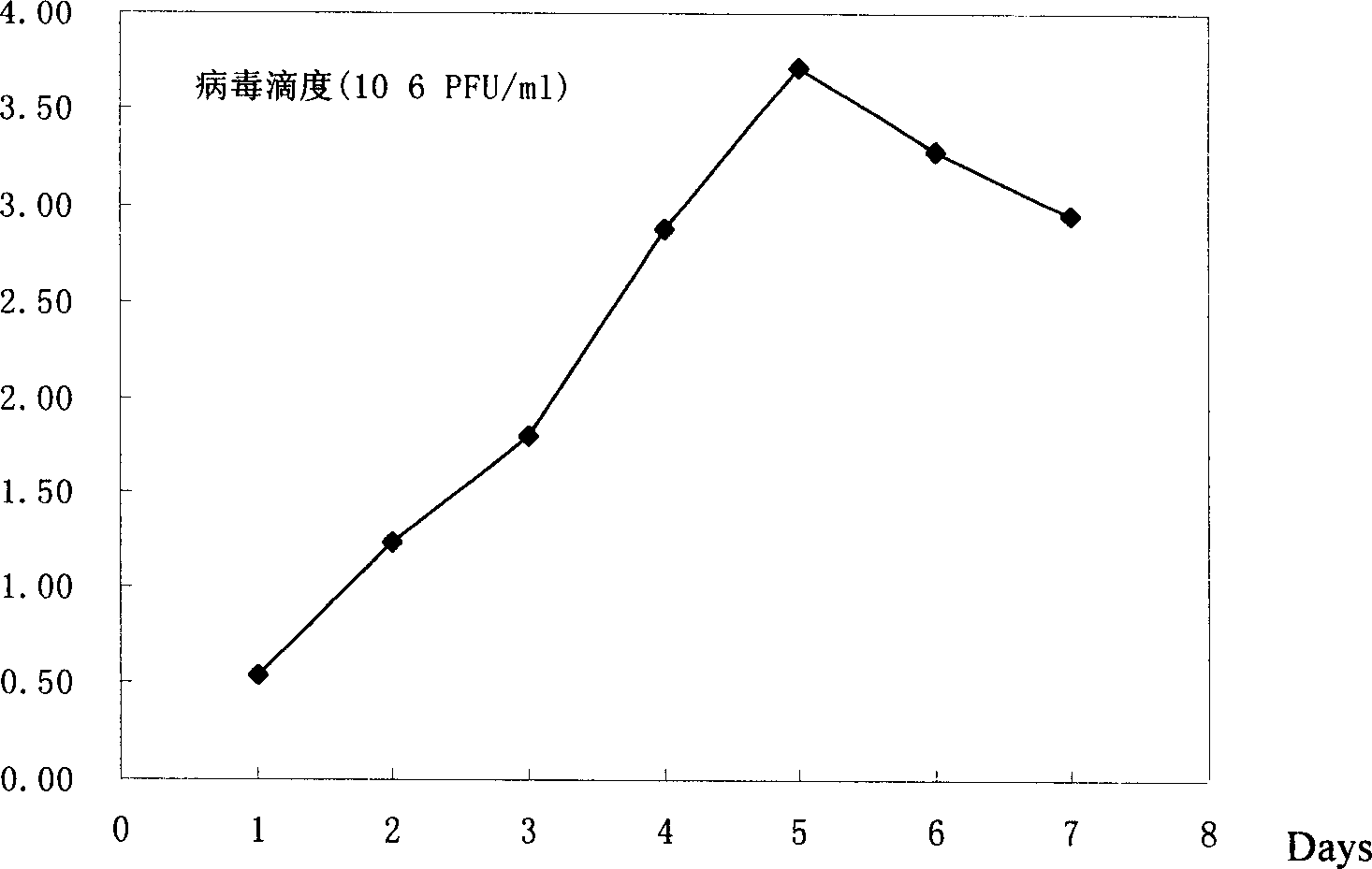
[0042] Vero cells (ATCC CCL81) in the logarithmic growth phase were cultured in DMEM-F12 supplemented with 10% FCS (Gibco Company) to form monolayer cells, and the culture area of the culture flask was 150 cm2. Prepare a single cell suspension after digestion with 0.25% trypsin, inoculate into a roller bottle, the initial cell concentration is 1×10 6 / ml, when the cells were cultured to be close to monolayer (80%), the culture medium was replaced with DMEM-F12 culture medium without 10% FCS and added with trypsin to a final concentration of 2 μg / ml. After the mixed virus strain preparation was treated with trypsin 20 μg / ml for 20 minutes, it was inoculated into a roller bottle, and the inoculated virus amount was 2×10 5 For TCID virus...
Embodiment 3
[0043] Embodiment 3: the preparation of rotavirus antigen
[0044] 1. Purification of Virus
[0045] 8L of virus suspension collected after in vitro amplification. Using a Millipore Pellicon ultrafiltration device, the ultrafiltration concentration was 10 times at 4°C, and the molecular weight cut-off was 30KD. The buffer was then switched using 50 mM Tris / HCl, pH=8.0.
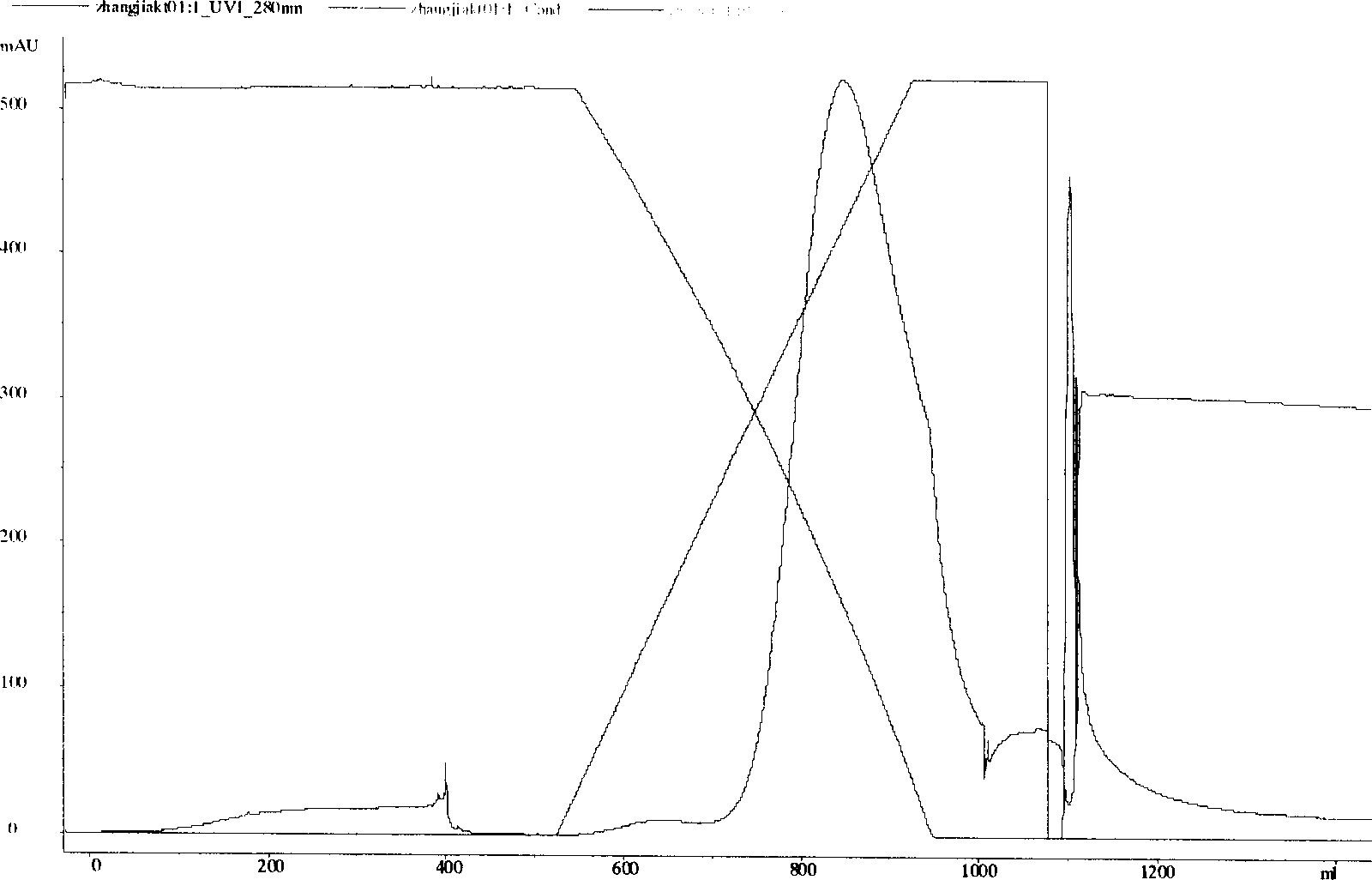
[0046] Load the above preparation to a DEAE-Sepharose FF column (2.6×10cm) equilibrated with 50mM Tris / HCl, pH=8.0 in advance, and after fully eluting unbound components, use a linear gradient of 0-0.5M NaCl to elute . Combine virus-containing components for later use.
[0047] 2. Virus inactivation
[0048] The concentrated and purified rotavirus preparation can be inactivated with formalin, phenol, glutaraldehyde, β-propiolactone and other inactivating agents before use. If formalin is used, the dosage between 0.01-0.5%, and the optimal concentration used in the present invention is 0.1%. The inactiva...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com