Oral hexavalent reassortment live rotavirus vaccine
A technology of rotavirus and live vaccines, which is applied in antiviral agents, virus antigen components, viruses/bacteriophages, etc., and can solve the problems of small serotype coverage and coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1. Preparation of each serotype virus stock solution
[0046] Cell passage: The culture substrate of each serotype virus is Vero cells. First, resuscitate the working seeds of Vero cells. After culturing at 37°C for 3-5 days, carry out passage and expansion culture according to a certain ratio. The culture containers are T25, T75, and T175 cells respectively. Culture flasks, 10-layer cell factories, and 40-layer cell factories were inoculated with G1, G2, G3, G4, G8, and G9 serotype rotavirus after culturing 3-5 generations in the 40-layer cell factory.
[0047] Virus culture: After the cell confluence in the cell factory reaches more than 90%, the virus inoculation operation can be carried out. Before virus inoculation, select a cell factory for cell counting. According to the counting results, calculate according to MOI (multiplicity of virus infection) 0.005-0.2 to obtain the required amount of virus, and activate the virus with trypsin. The activation co...
Embodiment 2
[0050] Embodiment 2. Development of vaccine protective agent
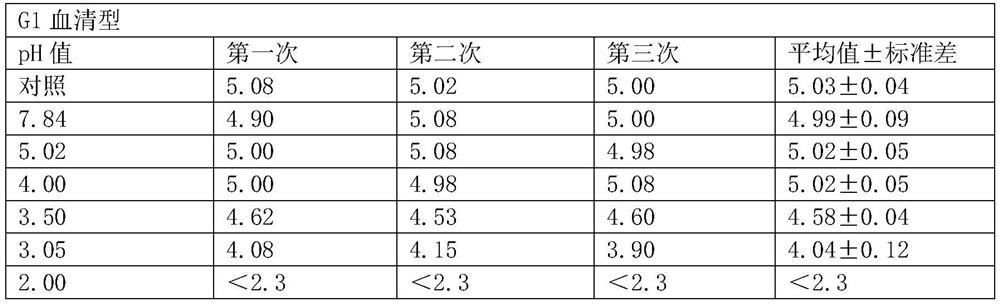
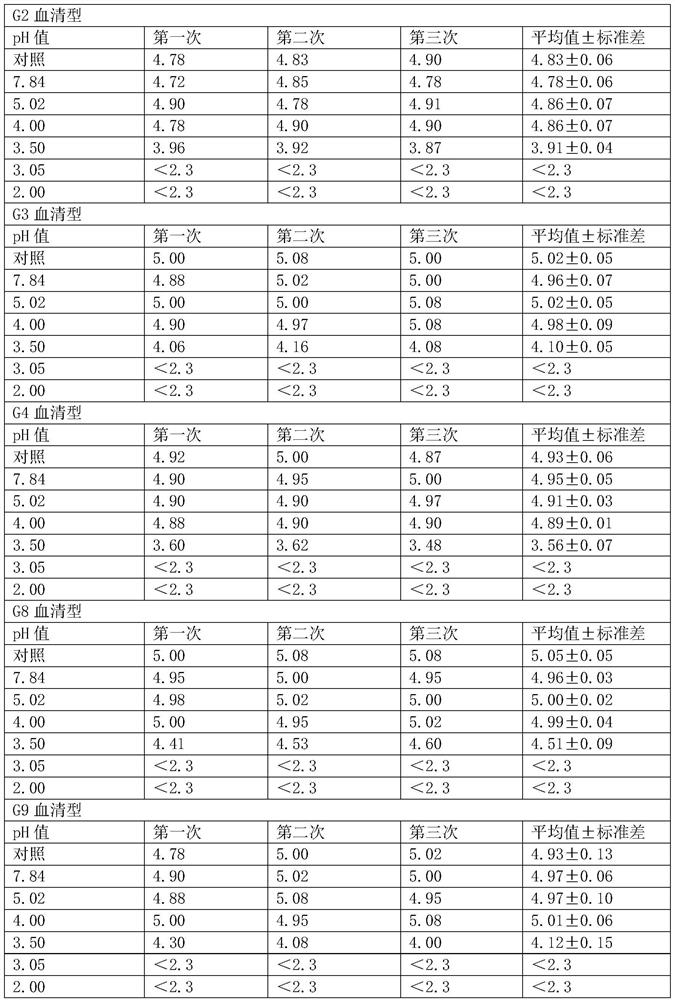
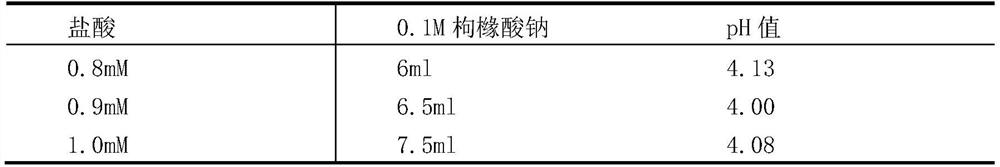
[0051] (1) Determination of virus resistance to acid
[0052] The minimum pH value that the hexavalent rotavirus vaccine can tolerate is determined by experiment.
[0053] Mix the hexavalent serotype virus mixed vaccine (the stock solution of the hexavalent vaccine is mixed in equal volumes) and 1% sodium citrate solution in equal volumes.
[0054] (2) Prepare 7 disposable sterile 50ml centrifuge tubes, 6 of which were added with 2ml of hexavalent seedlings mixed in step (1), and the other 1 was added with 1ml of hexavalent mixed seedlings and 1ml of DMEM as a control.
[0055] (3) To adjust the pH, add 0.1M hydrochloric acid 0ml, 0.4ml, 0.6ml, 0.7ml, 0.8ml, 1.2ml to 6 centrifuge tubes respectively, and the corresponding pH values are 7.84, 5.02, 4.00, 3.50, 3.05, 2.00 .
[0056] (4) Place 7 centrifuge tubes in a 37°C water bath and incubate for 2 hours.
[0057] (5) After the incubation time is over, immedia...
Embodiment 3
[0085] Example 3. Screening of different formulations
[0086] See Table 4 for the composition contents of 5 kinds of vaccine protective agent formulations
[0087] The content of each component of different protective agents in table 4
[0088]
[0089]
[0090] Each formula was mixed with the most temperature-sensitive serotype G9 virus, and an accelerated stability test was carried out. The results are shown in Table 5
[0091] The virus stability test of table 5 different protection formulations (all data are the mean value of three detection results, unit: lgFFU / ml)
[0092]
[0093] It can be seen that the protection of the above five formulas to G9 all meets the requirements, among which F5 is stored at 37°C for 7 days, the drop in G9 titer is the lowest, and the protection to G9 is the best, so F5 is the best for rotavirus vaccine Preservative formula.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com