Rotavirus strain and application thereof as vaccine
A technology of sequences and amino acids, applied in the field of rotavirus strains and their use as vaccines, can solve the problems that the protection effect of vaccines needs to be improved, pollution, and cannot be used for immunodeficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
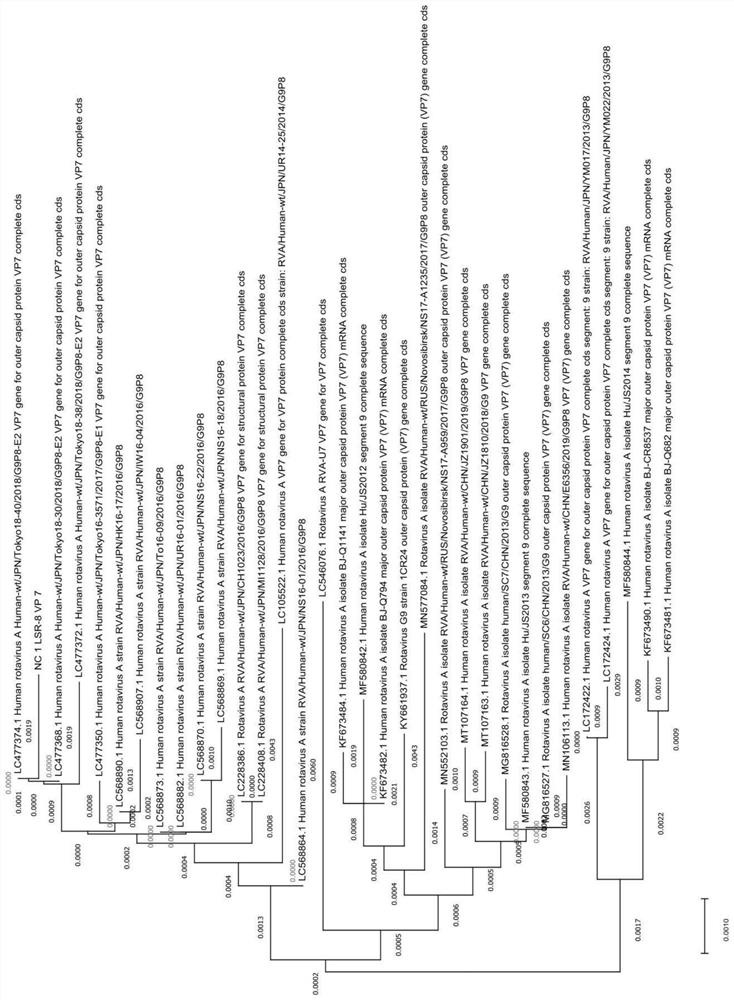
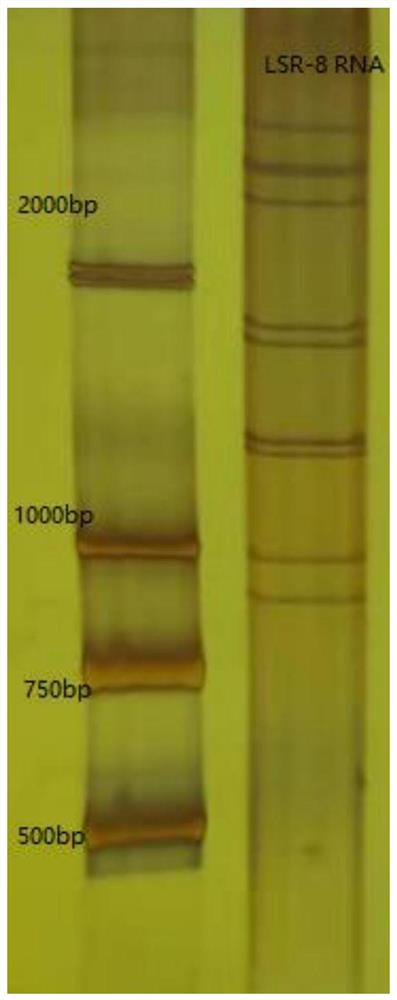
[0120] Isolation and characterization of embodiment 1LSR-8 strain
[0121] Get a virus sample
[0122] Collect diarrheal excrement samples from children with pediatric diarrhea in the hospital, resuspend in 0.01M PBS solution with pH 7.2, centrifuge at 8000rpm and 4°C for 20 minutes to get the supernatant, and test positive for rotavirus by colloidal gold kit, use 0.22 Sterilize and filter with a μm filter, aliquot into 1.5ml sterile centrifuge tubes, and store at -80°C for later use.
[0123] Plaque purification of virus morphology of LSR-8 strain
[0124] Add trypsin to the rotavirus harvest solution and activate it in a constant temperature water bath at 37°C for 1 hour, then dilute to 10 times with maintenance solution 10 times. -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 .
[0125] MA104 cells were inoculated into a 6-well plate, and when the cells grew into a dense monolayer, the growth medium was discarded, and the cell surface was washed 3 times with ...
Embodiment 2
[0138] Adaptability and passage of embodiment 2LSR-8 strain
[0139] Adaptation of LSR-8 Strain Virus on VERO Cells
[0140] Add trypsin to the rotavirus harvest solution and activate it in a constant temperature water bath at 37°C for 1 hour.
[0141] Take the VERO cells that have grown into a dense monolayer in the T25 flask, discard the growth medium, and wash the cell surface with the maintenance solution. Aspirate the remaining maintenance solution, add 500ul of activated rotavirus solution, absorb at 37°C for 60-120 minutes, add 10ml of maintenance solution containing trypsin with a final concentration of 4μlg / ml, and incubate at 37°C, 5% CO 2 Cultured in medium to observe the cytopathic effect CPE.
[0142] When cells above 75% CPE appeared, virus was harvested. Freeze and thaw the harvested virus culture solution twice at -20°C and 25°C alternately, centrifuge at 8000 rpm and 4°C for 20 minutes, take the supernatant, and inoculate VERO cells for passage in the same ...
Embodiment 3
[0143] The identification method of embodiment 3LSR-8 strain
[0144] LSR-8 strain virus titer determination:
[0145] Add trypsin to the rotavirus harvest solution and activate it in a constant temperature water bath at 37°C for 1 hour, then dilute it to 10 times with the maintenance solution. -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 .
[0146] MA104 cells were inoculated into a 6-well plate, and when the cells grew into a dense monolayer, the growth medium was discarded, and the cell surface was washed 3 times with maintenance medium. Aspirate the remaining maintenance solution, add 500 μl of activated rotavirus solution, absorb at 37°C for 60-120 minutes, discard the virus solution, add 3ml of maintenance solution containing 0.3% agar, each dilution gradient is 2 wells, wait After the agar has solidified, transfer it to the incubator. After 72 hours, a maintenance solution containing 0.006% neutral red and 0.3% agar was covered on the original agar laye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com