Quadruple fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for detecting porcine epidemic diarrhea virus and porcine rotavirus and application of quadruple fluorescent quantitative PCR detection kit
A technology of porcine epidemic diarrhea and porcine rotavirus, applied in the biological field, can solve the problems of high harm, high infectivity and pathogenicity of diarrhea pathogens, and achieve the best specificity and repeatability, excellent sensitivity, and reduce workload Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A quadruple fluorescence quantitative PCR detection kit for the detection of porcine epidemic diarrhea virus and porcine rotavirus, including hot-start Taq DNA polymerase, enzyme-free water, PCR reaction solution, and probe-based fluorescence quantitative PCR matching Buffer, And four pairs of specific primers and corresponding TaqMan probes and controls for the detection of G1 genotype porcine epidemic diarrhea virus, G2 genotype porcine epidemic diarrhea virus, porcine group A rotavirus, and porcine group C rotavirus ; wherein, the PCR reaction solution contains Mg 2+ ions, PCR buffer, dNTPs mix.
[0046] The control substance includes positive control substance and negative control substance: the positive control substance is the S gene fragment of G1 genotype porcine epidemic diarrhea virus and G2 genotype porcine epidemic diarrhea virus, porcine group A rotavirus and porcine epidemic diarrhea virus respectively. Four target genes of VP6 gene fragment of porcine gr...
Embodiment 2 4
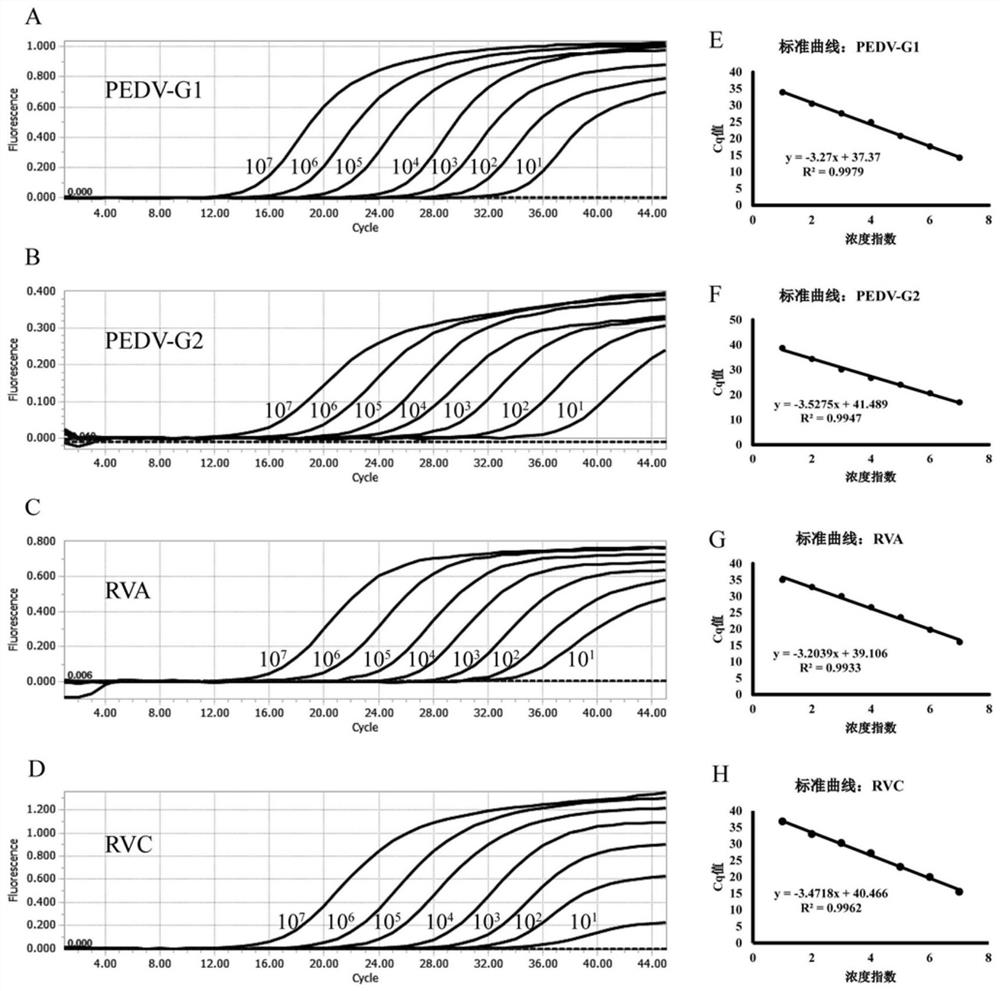
[0078] Example 2 Establishment of standard curve of quadruple TaqMan qPCR detection method
[0079] 1. All clinical samples are stored in -80℃ low temperature refrigerator. The clinical samples were freeze-thawed and placed in a 1.5 mL centrifuge tube, PBS buffer was added and ground sufficiently, and then centrifuged at 12,000 rpm for 10-15 min to collect the sample supernatant. The viral nucleic acid was extracted from the supernatant according to the instructions of the nucleic acid extraction kit Viral DNA / RNA Kit.
[0080] 2. Using the extracted viral nucleic acid as a template for reverse transcription, use the RevertAid First StrandcDNA Synthesis Kit to reverse-transcribe the viral nucleic acid into cDNA. The total reaction volume is 20 μL, including: 1 μL RandomHexamer Primer, 1 μL Oligo(dT) 18Primer, 5μL nuclease-free Water, 5μL total RNA, incubate at 65°C for 5min after mixing, ice bath for 3min, continue to add: 4μL 5×Reaction Buffer, 2μL 10×dNTP master mix, 1μLRev...
Embodiment 3 4
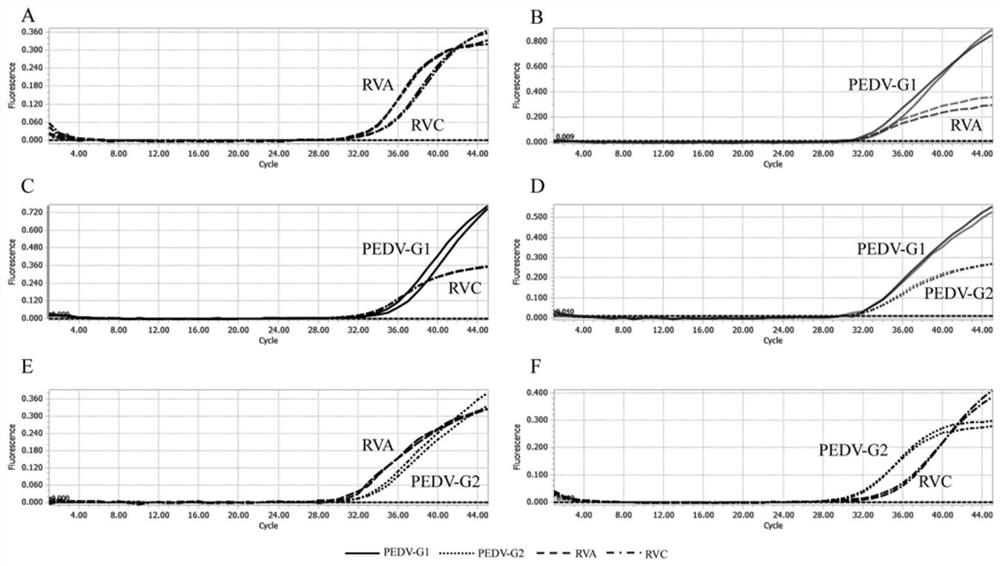
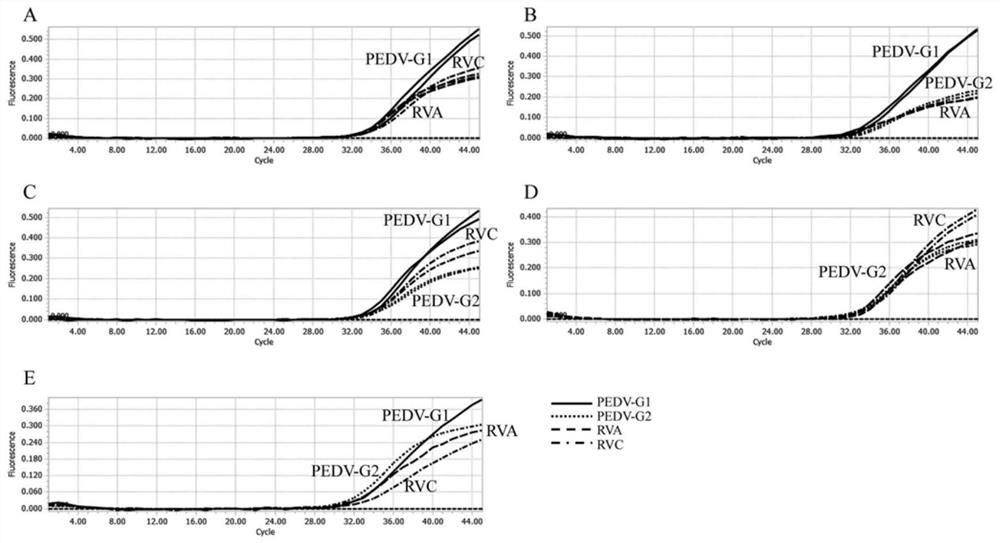
[0088] Example 3 The quadruple TaqMan qPCR detection method is most suitable for the exploration of the reaction system
[0089] 1. Use a concentration of 1×10 4 copies / μL of PEDV-G1, PEDV-G2, RVA and RVC plasmid standards were placed into the qPCR reaction system, each system used primers of different concentrations, PCR amplification was performed, and fluorescent signals were collected.
[0090] 2. The optimal primer concentration qPCR reaction system is: 10 μL Probe Master Mix (containing Mg 2+ ions, dNTPs mixture, hot-start Taq DNA polymerase, etc.), 0.1 μL each of PEDV-G1-Probe, PEDV-G2-Probe, RVA-Probe and RVC-Probe, 2 μL cDNA template, nuclease-free water was added to the total volume of the system 20 μL, PEDV-G1(F), PEDV-G1(R), PEDV-G2(F), PEDV-G2(R), RVA(F), RVA(R), RVC(F) and RVC(R) 0.1 μL, 0.2 μL, 0.3 μL, 0.4 μL, 0.5 μL, 0.6 μL, 0.7 μL, 0.8 μL were added, respectively.
[0091] 3. The optimal probe concentration qPCR reaction system is: 10 μL Probe Master Mix (c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com