Multi-PCR (polymerase chain reaction) rapid diagnostic kit for five porcine diarrhea viruses and application of the multiple PCR rapid diagnostic kit
A rapid diagnosis and kit technology, applied in the direction of resistance to vector-borne diseases, microbiological determination/inspection, biochemical equipment and methods, etc., can solve problems such as difficult early detection, low sensitivity, cumbersome operation, etc., to reduce the number of pigs Population mortality and economic loss, high specificity, low time saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, kit structure
[0049] A multiplex real-time fluorescent quantitative PCR rapid diagnostic kit for five kinds of porcine viruses, consisting of multiplex PCR reaction mixture, virus primers, TGEV virus standard, GAR virus standard, GCR virus standard, PEDV virus, PCV2 virus standard, positive control Products, negative controls and boxes.
[0050] There are corresponding container holes in the box body, which are used to place quantitative PCR reaction solution tubes, five virus primer tubes, TGEV virus standard tubes, GAR virus standard tubes, GCR virus standard tubes, and PEDV virus standard tubes respectively. tube, PCV2 virus standard tube, positive control tube and negative control tube.
[0051] The multiplex PCR reaction mixture contains PCR reaction buffer (containing magnesium chloride and deoxyribonucleotide triphosphate mixture, etc.) and heat-resistant DNA polymerase; the viral primers are five kinds of viral primers, the upstream and downstre...
Embodiment 2
[0052] Embodiment 2, the present invention detects five kinds of virus-specific experiments
[0053] Step 1: Primer Synthesis
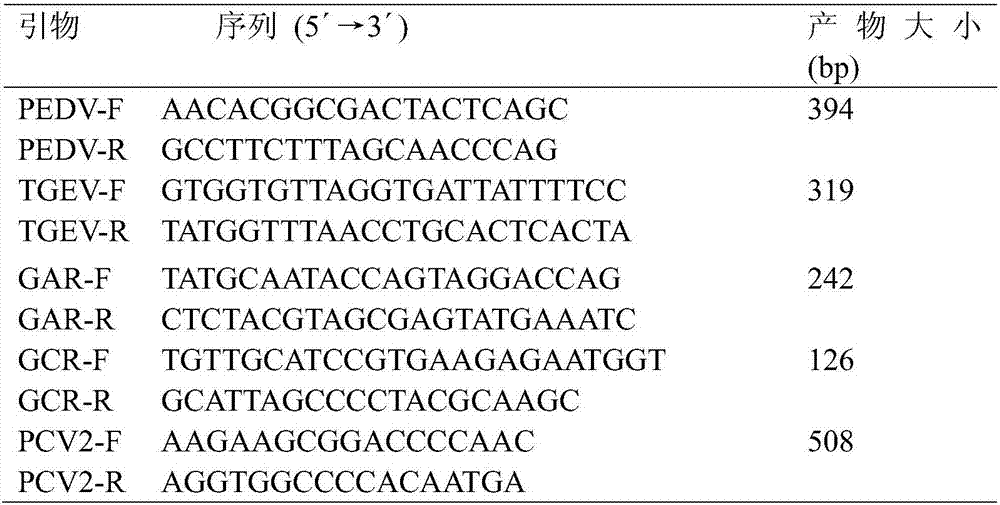
[0054] The primer sequences of 5 kinds of viruses designed in the present invention (see Table 1) were synthesized by China Shanghai Sangon Biological Company Technical Service Company, and the synthesis amount was 3 OD per primer.
[0055] Step 2: Specific detection of multiplex PCR system
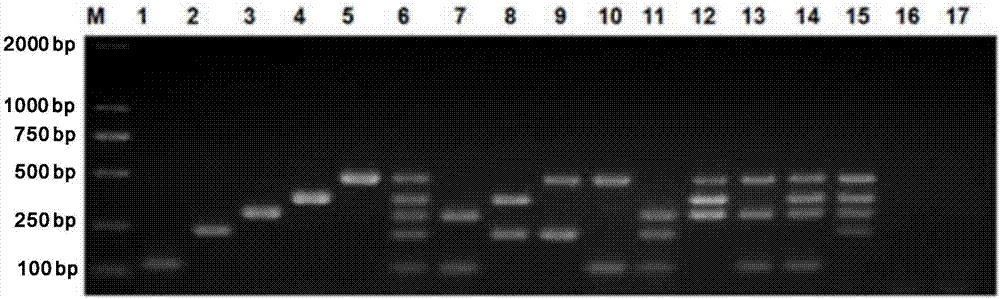
[0056] According to the multiplex PCR reaction system, take 17 copies of the same PCR reaction master mix (i.e., 19 μL of multiplex PCR reaction mixture and 1 μl of 5 virus primer mixtures), and add 1 μL of positive sample cDNA / DNA template 1: GCR; 2: GAR; 3: TGEV; 4: PEDV; 5: PCV2; 6: GCR, GAR, TGEV, PEDV and PCV2; 7: GCR and TGEV; 8: GAR and PEDV; 9: TGEV and PCV2; 10: GCR and PCV2; 11: GCR, GAR, and TGEV; 12: TGEV, PEDV, and PCV2; 13: GCR, TGEV, and PCV2; 14: GCR, TGEV, PEDV, and PCV2; 15: GAR, TGEV, PEDV, and PCV2; 16: Deionized water negative control ;17:...
Embodiment 3
[0057] Embodiment 3, the present invention detects five kinds of virus sensitivity experiments
[0058] Step 1: Primer Synthesis
[0059] With the first step in embodiment 2.
[0060] Step 2: Sensitivity Testing
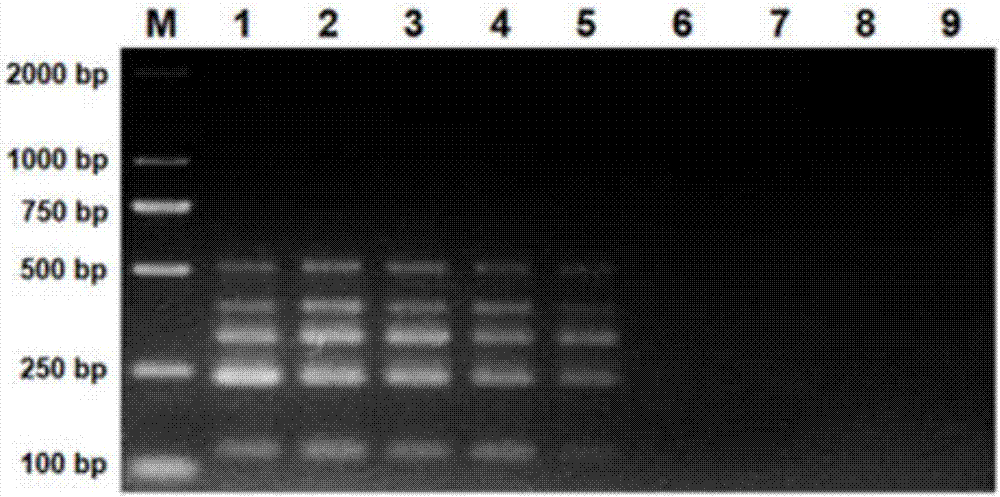
[0061] The multiplex PCR sensitivity detection system is as follows: 19 μL PCR reaction solution (multiple PCR reaction mixture), 10 μM TGEV, GAR, GCR, PEDV and PCV2 upstream and downstream primer mixture 1 μL, 1 μL 10-fold serial dilution of TGEV, GAR, GCR, PEDV and PCV2 plasmid standard (5.0×10 8 ~5.0×10 1 copies / μL) template, add ddH 2 O to the total volume of the reaction system was 25 μL; the reaction was carried out in a Bio-Rad S1000 PCR amplification instrument, and the reaction parameters were denaturation at 95°C for 3 minutes; 40 cycles of denaturation at 95°C for 20 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s; the last 72 ℃ 5min. Take 5 μL of PCR product, mix with 1 μL LoadingBuffer, and detect by 2% agarose electrophoresis.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com