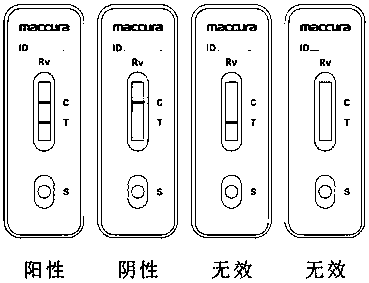
Hybridoma Cells Secreting Murine Anti-group A Rotavirus Monoclonal Antibody and Its Secreted Monoclonal Antibody
A technology of monoclonal antibody and hybridoma cells, which is applied in the field of hybridoma cells, can solve the problems of insufficient stability and reliability of detection results, misplacement of reagents, etc., and achieve high sensitivity, strong specificity, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation and screening of hybridoma cell lines
[0014] (1) RV antigen immunized mice
[0015] RV antigen (purchased from Hangzhou Qitai Biotechnology Co., Ltd.) was diluted to 2.0 mg / ml with normal saline, mixed with an equal volume of Freund's complete adjuvant (purchased from Sigma, product number SLBF-9338V), and emulsified into Oily emulsion, the emulsification can not be stopped until the oily emulsion dropped into water does not disperse. The emulsion was subcutaneously administered to BALB / c mice (purchased from Chengdu Dashuo Experimental Animal Center, 6-week-old female, 2 mice) in the armpits of limbs at a dose of 100 μl / mouse. Immunization was boosted 14 days after the first immunization, the antigen was mixed with incomplete Freund's adjuvant (purchased from Sigma, product number SLBM9367V) in equal volumes and then emulsified, and the immunization dose was 50 μl per mouse. Immunization was boosted every other week, tail blood was collected before ea...
Embodiment 2
[0028] Preparation of monoclonal antibodies
[0029] Select 6-8 weeks healthy BALB / c mice, inject 0.5mL liquid paraffin (Tianjin Kemiou Chemical Reagent Co., Ltd.) into each mouse intraperitoneally, and inject 0.5~1×10 6 A hybridoma cell line RVAb-1 or a hybridoma cell line RVAb-2. Ascites was produced 7-10 days after cell inoculation, and the health status and signs of ascites of the mice were closely observed. When there was as much ascites as possible, and before the mice were about to die, the mice were killed, and the ascites was sucked into the test tube with a dropper. Collect 8mL and 10mL ascites. The collected ascites was centrifuged at 4000rpm for 10min, the supernatant was taken, and small samples were taken respectively and stored in a -20°C refrigerator. The ascitic fluid was saturated and precipitated with ammonium sulfate, and then purified with a protein A affinity chromatography column. The purity of the two monoclonal antibodies detected by SDS-PAGE was g...
Embodiment 3
[0031] Potency detection
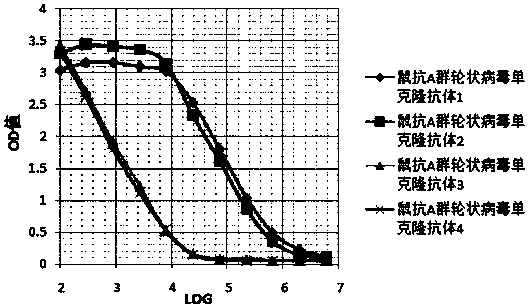
[0032] (1) Titer detection of hybridoma cell culture supernatant
[0033] RV antigen (Hangzhou Qitai Biotechnology Co., Ltd.) was diluted to 5 μg / ml with 0.06M pH9.6 carbonic acid buffer solution, and 100 μl was coated on each well of a 96-well microtiter plate. Place in the refrigerator at 2-8°C overnight, discard the liquid in the well the next day, wash the ELISA plate washing machine three times, pat dry, and use 0.01M PBS pH7.2 containing 10% calf serum, 150μl / well, 37°C After blocking for 2 hours, the liquid was discarded and patted dry to detect the titer of hybridoma cell culture supernatant, ascites fluid, and purified antibody. Hybridoma cell culture supernatant titer detection The first well is the original supernatant culture solution, from the second well to the fourth well with 0.01M pH7.2 PBS buffer 10-fold serial dilution, the fifth to the tenth well Wells were serially diluted 2-fold. In the eleventh well, the mouse serum dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com