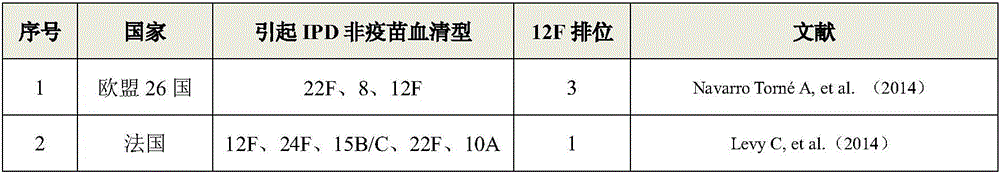
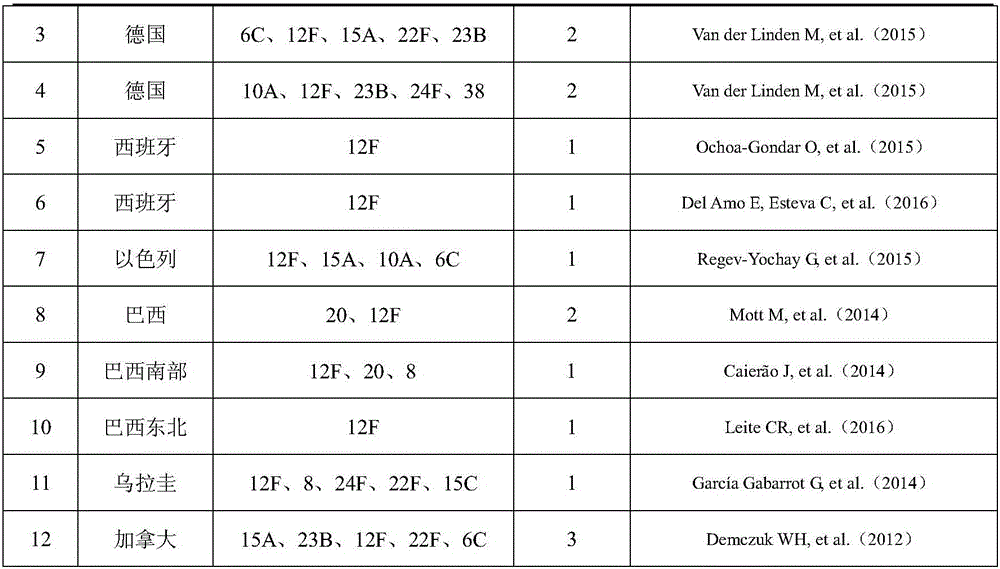
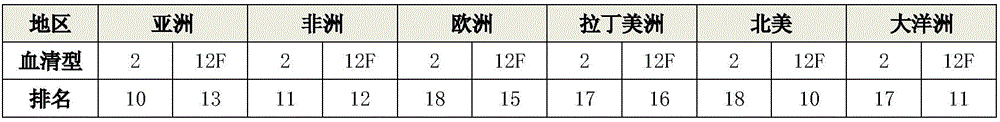
15-valence pneumococcus conjugate combined vaccine containing 2-type and 12 F serotypes
A pneumococcal and combined vaccine technology, applied in the direction of multivalent vaccines, vaccines, antibacterial drugs, etc., can solve the problems of high lethality and non-inclusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: 15-valent pneumococcal conjugate combination prescription
[0037] Each 0.5ml dose contains a total of 32 μg of various types of polysaccharides (total polysaccharides), including 4 μg of type 6B, 2 μg each of type 1, 2, 3, 4, 5, 6A, 7F, 9V, 12F, 14, 18C, 19A, 19F, and 23F . Various types of polysaccharides are combined with diphtheria toxoid and adsorbed on aluminum phosphate adjuvant (aluminum content 0.125 mg), and the solvent is physiological sodium chloride solution.
Embodiment 2
[0038] Example 2: 15-valent pneumococcal conjugates combined with different injections of antibodies after immunizing mice
[0039] NIH mice were used to subcutaneously immunize 3 injections, each injection was separated by 2 weeks, and the dose was 0.1ml. Blood was collected 14 days after each dose of immunization, serum was separated, and the specific serum titer was determined. The serum antibody titer was determined by taking the mean value of the negative control plus 2 times SD as the cutoff value, and the data was analyzed after taking the logarithm, and the seroconversion rate was evaluated by the increase of the antibody by more than 4 times. The results show that the conjugate combination of the present invention can induce high-titer specific antibodies in mice, and ideal antibody titers and seroconversion rates can be achieved with one shot of immunization. For mice with very high antibody titers, again Antibody titers after immunization no longer increased.
[0...
Embodiment 3
[0042] Example 3: 15-valent pneumococcal conjugate combination guinea pig active allergy test
[0043] Divided into two immunization dose groups of 2ml and 0.5ml, healthy white guinea pigs were intraperitoneally injected with 15-valent pneumococcal combination combination, once every other day, for sensitization for 3 consecutive times; on the 12th day after the last sensitization, a single intravenous injection was used to sensitize twice The combination of 15-valent pneumococcal conjugates of the dose was used for stimulation, and the allergic reaction of the 2ml dose group to guinea pigs was positive (++3, +++2, ++++1), and the main symptoms were restlessness, piloerection, Trembling, nose scratching, sneezing, coughing, shortness of breath, urination, tearing, dyspnea, unsteady gait, convulsions, death, the reaction appeared about 1 minute after administration, and death appeared about 5 minutes after administration, the surviving mice The reaction disappeared within half ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com