Treatment of spinal muscular atrophy
A technology for spinal muscular atrophy and treatment methods, applied in the field of recombinant adeno-associated virus vectors, which can solve the problems of exon 7 skipping and low protein efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
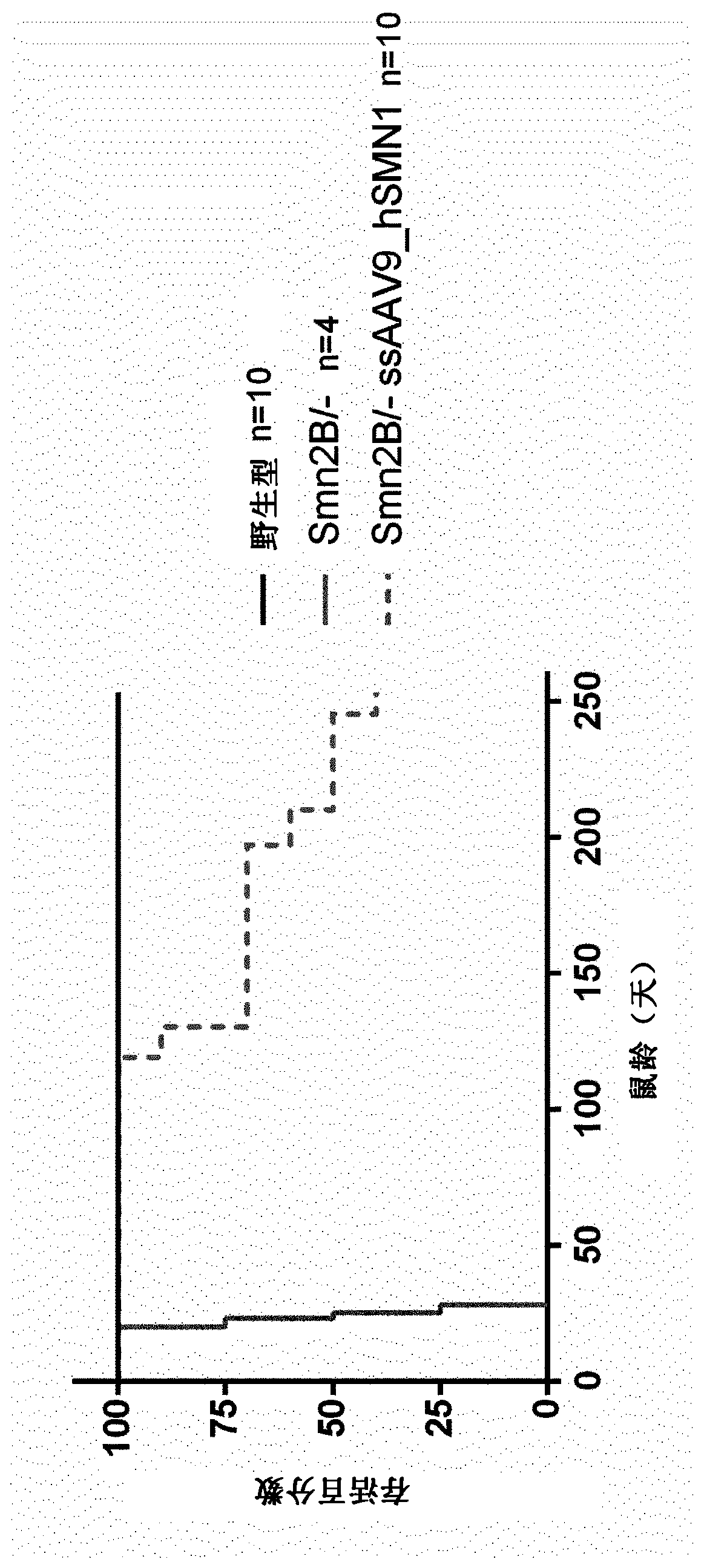
[0256] It is demonstrated herein that survival of a mouse model of SMA is greatly improved beyond expectations following administration of an AAV vector carrying the human SMN1 gene in the single-stranded genome.
[0257] Materials and methods
[0258] Vector production
[0259] The AAV vector used was a single-chain recombinant AAV9 vector with human SMN1 under the control of the CAG promoter (hybrid CMV enhancer / chicken β-actin promoter and β-globin splice acceptor site) gene, and the polyA region from the HBB2 gene.
[0260] The ssAAV9 vector was produced by a triple transfection system using standard procedures (Xiao et al., J. Virol. 1998; 72:2224-2232). Pseudotyped recombinant rAAV2 / 9 (rAAV9) virus preparations were generated by packaging the AAV2-terminal inverted repeat (ITR) recombinant genome in an AAV9 capsid. Briefly, a cis-acting plasmid carrying the CAG-hSMN1 construct, a trans-complementing rep-cap9 plasmid encoding proteins essential for the replication an...
Embodiment 2
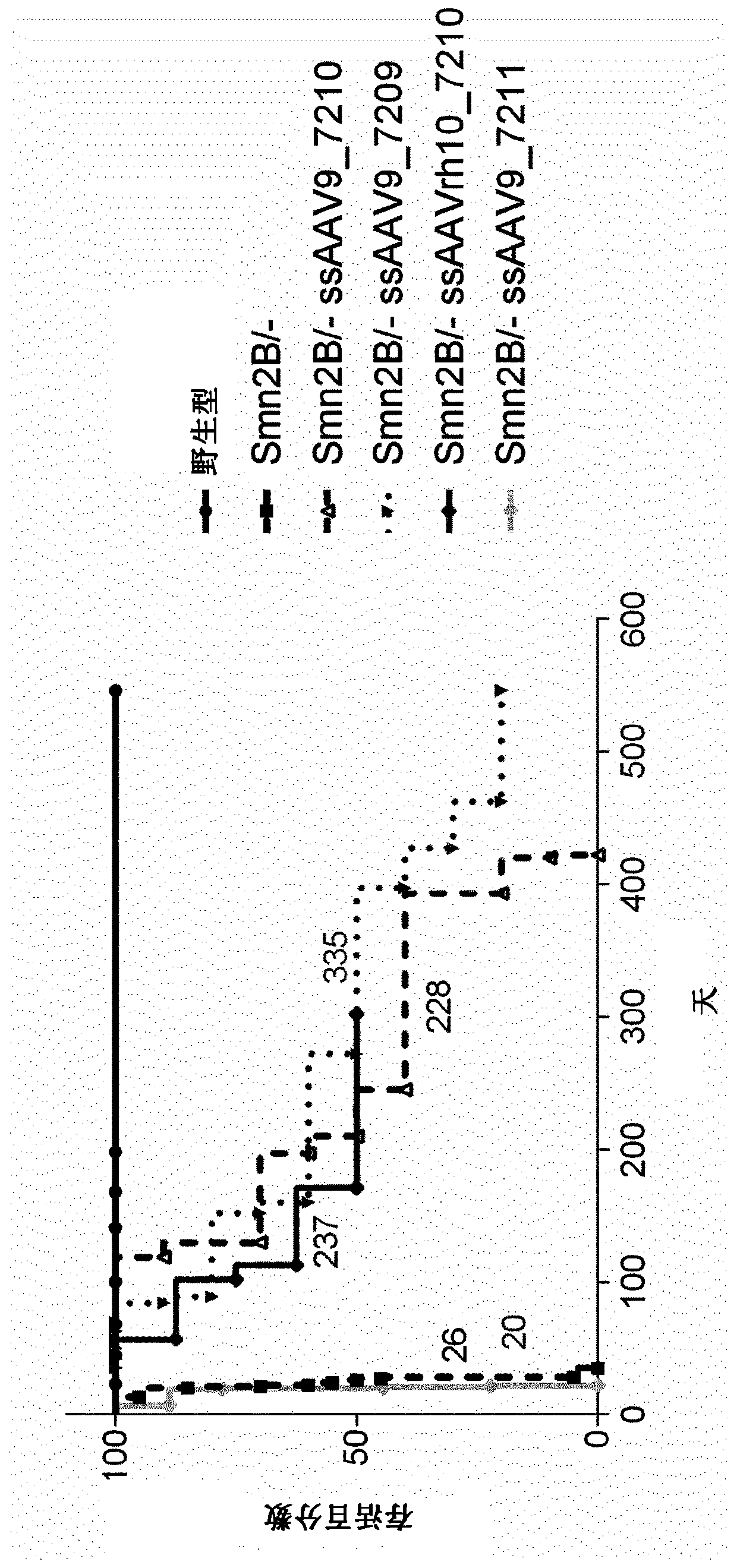
[0270] Additional experiments were performed to show the improvement obtained using the present invention.
[0271] The aim of this study was to evaluate the therapeutic efficacy of a single-chain (ss) AAV9 vector expressing human SMN1 in a mouse model of spinal muscular atrophy. We compared intraventricular (ICV) administration to Smn 2B / - Effects of three ssAAV9-hSMN1 vectors and one ssAAVrh10-hSMN1 vector in neonatal mice at 21 and 90 days post-injection.
[0272] We analyzed different parameters:
[0273] - lifetime,
[0274] -weight,
[0275] - exercise and muscle strength,
[0276] - vector biodistribution and transgene expression,
[0277] - human SMN protein expression in various tissues,
[0278] - Spinal motor neuron counts,
[0279] - skeletal muscle histology,
[0280] - Neuromuscular junction (NMJ) morphology.
[0281] Three ssAAV9-hSMN1 vectors (7209, 7210, and 7211) containing wild-type human SMN1 coding sequence (NCBI reference sequence: NM_000344.3) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com