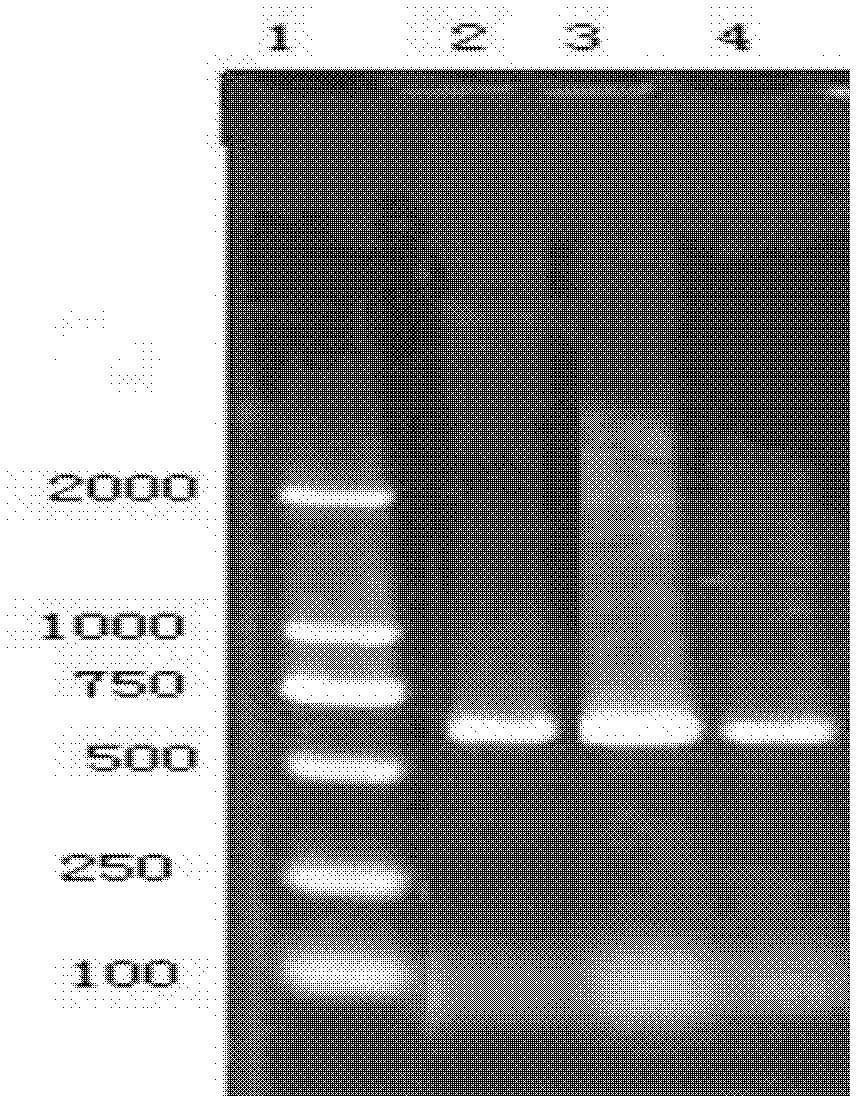Porcine contagious pleuropneumonia trivalent inactivated vaccine and its preparation method
A technology of porcine pleuropneumonia and pleuropneumonia, applied in the field of veterinary drug vaccines, can solve the problems of fever and easy residue formation, and achieve the effects of small immune stress, good safety, and avoiding oil residue problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
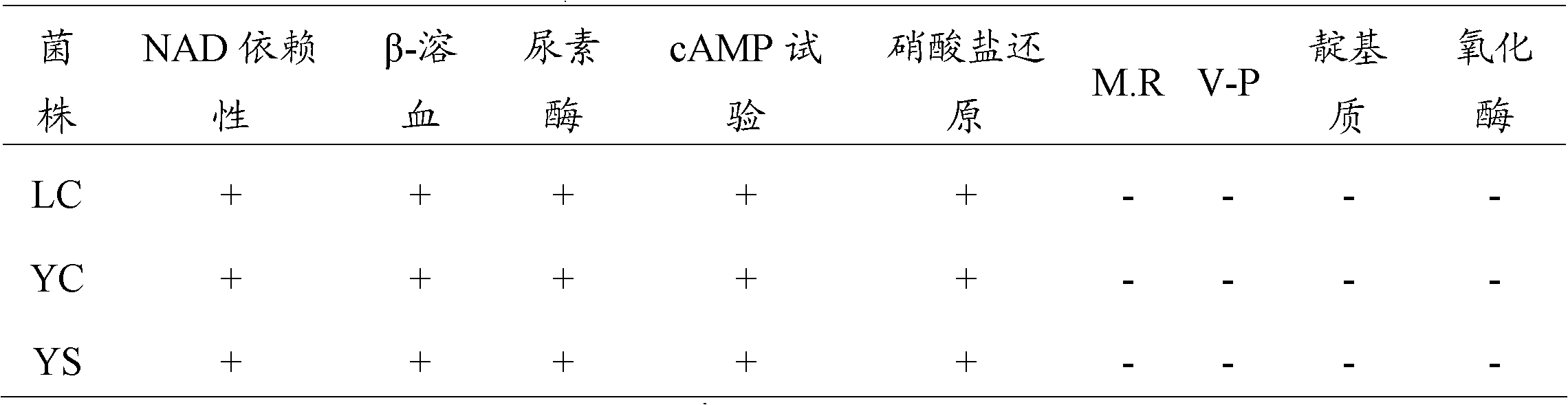
[0036] The identification of the isolation of embodiment 1 serotype 1 LC strain, 5 type YC strain, 7 type YS strain
[0037] 1 Materials and methods
[0038] 1.1 The disease materials to be tested were collected from 10 pig farms in Henan, Hubei and other provinces and cities with suspected pleuropneumonia.
[0039] 1.2PCR primer sequence
[0040] PF-5'CCGACTTTTAAATCCGT3', PR-5'GAACAGTTGTTCGCTAA3'
[0041] 1.3 Mice were purchased from Animal Experiment Center of Henan Province.
[0042] 1.4 Isolation and purification of pathogenic bacteria and liquid culture Aseptically take lungs, throat tonsils and other tissues of dying pigs, inoculate them on TSA (containing 1% NAD) agar medium, place 10% CO2, culture at 37°C for 24-36 hours, and then select typical A single colony was cultured for purification. The purified single colonies were selected and inoculated in TSB (containing 1% NAD) liquid culture medium, and cultured overnight on a shaker (180 r / min) at 37°C.
[0043] 1....
Embodiment 2
[0060] Example 2: Preparation and efficacy test of porcine pleuropneumonia trivalent inactivated vaccine
[0061] 1 Materials and methods
[0062] 1.1 Culture medium: Actinobacillus pleuropneumoniae culture medium was cultured with Tryptic Soy Broth (TSB) and Tryptic Soy Agar Medium (TSA), purchased from BD Difco Company, batch number 0169092. The preparation process was carried out according to the instructions, and all the prepared TSA and TSB medium contained 1% NAD.
[0063] 1.2 Preparation of production seeds
[0064] Take out 1 bottle each of the basic seeds of Actinobacillus pleuropneumoniae serum type 1, 5, and 7, inoculate them on TSA medium by streaking, culture at 37°C for 24 hours, select 6-8 typical colonies and mix them in TSB broth, Inoculate the TSA slant, incubate at 37°C for 24 hours, and store in a refrigerator at 4°C, according to Appendix 15 of "The Veterinary Pharmacopoeia of the People's Republic of China" (2005 Edition). Those that pass the inspectio...
Embodiment 3
[0090] Example 3 Immune Effect and Comparative Test of Porcine Infectious Pleuropneumonia Trivalent Inactivated Vaccine
[0091] 1 Materials and methods
[0092] 1.1 Vaccines
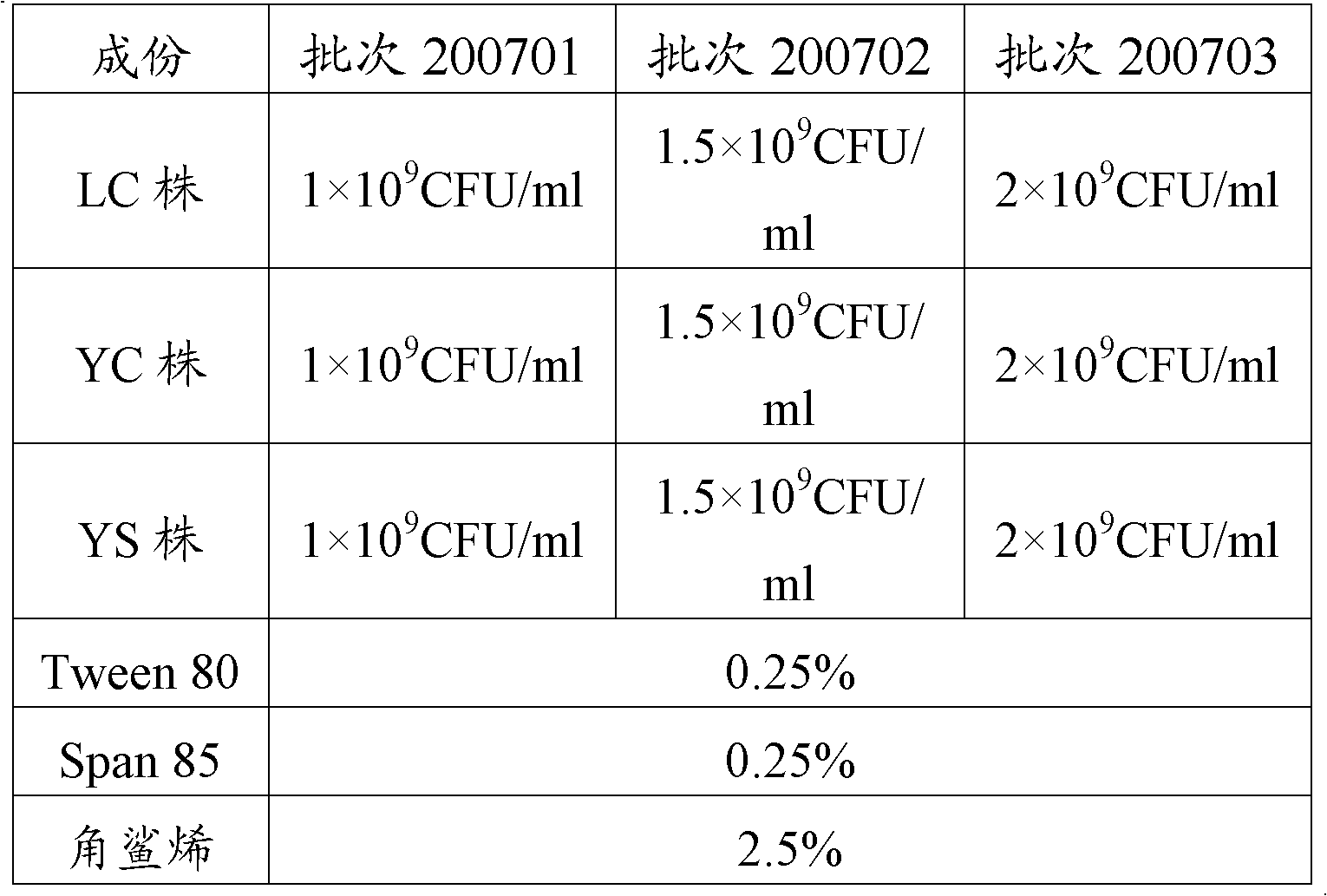
[0093] Three batches of porcine infectious pleuropneumonia trivalent inactivated vaccines prepared according to Example 2, batch numbers: 200701, 200702, 200703, self-made oil emulsion inactivated vaccines as control vaccines, containing 1, 5, 7 strains LC, YC, YS strain.
[0094] Preparation of oil emulsion inactivated vaccine: prepare type 1, 5, and 7 strain antigens according to the antigen preparation method in Example 2, and mix 3 kinds of antigens after passing the inspection. After mixing, the content of each antigen is 5 × 10 9 CFU / ml, stored at 4°C for future use. Add 4% Tween-80 to the water phase (antigen), mix the water phase and the oil phase at a ratio of 2:3, and emulsify with a homogenizer. , Dispense the emulsified vaccine into sterilized vaccine bottles as the control vaccine. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com