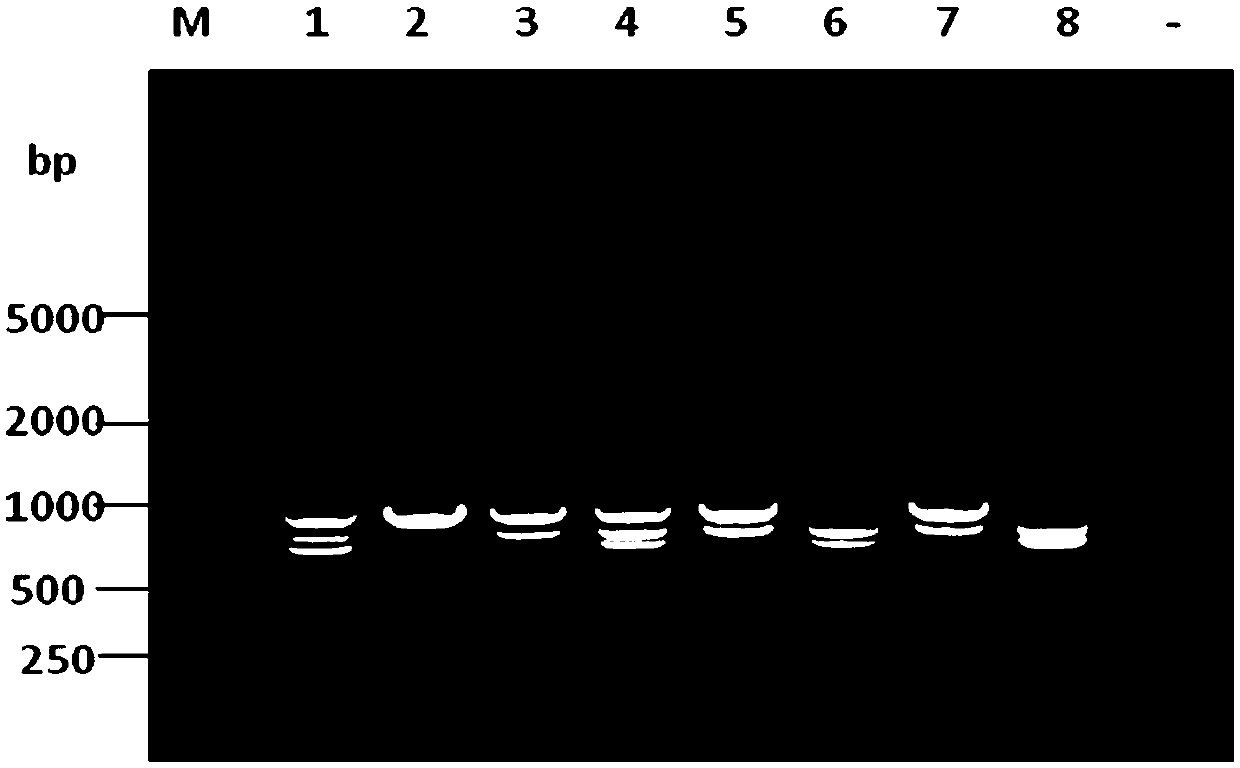
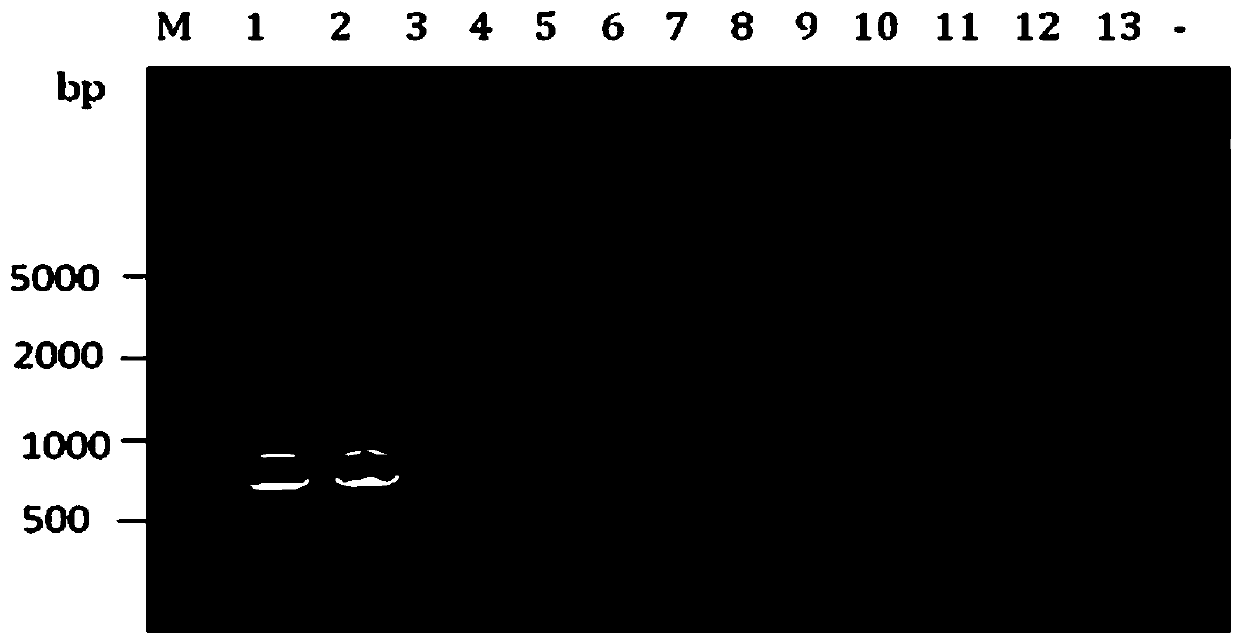
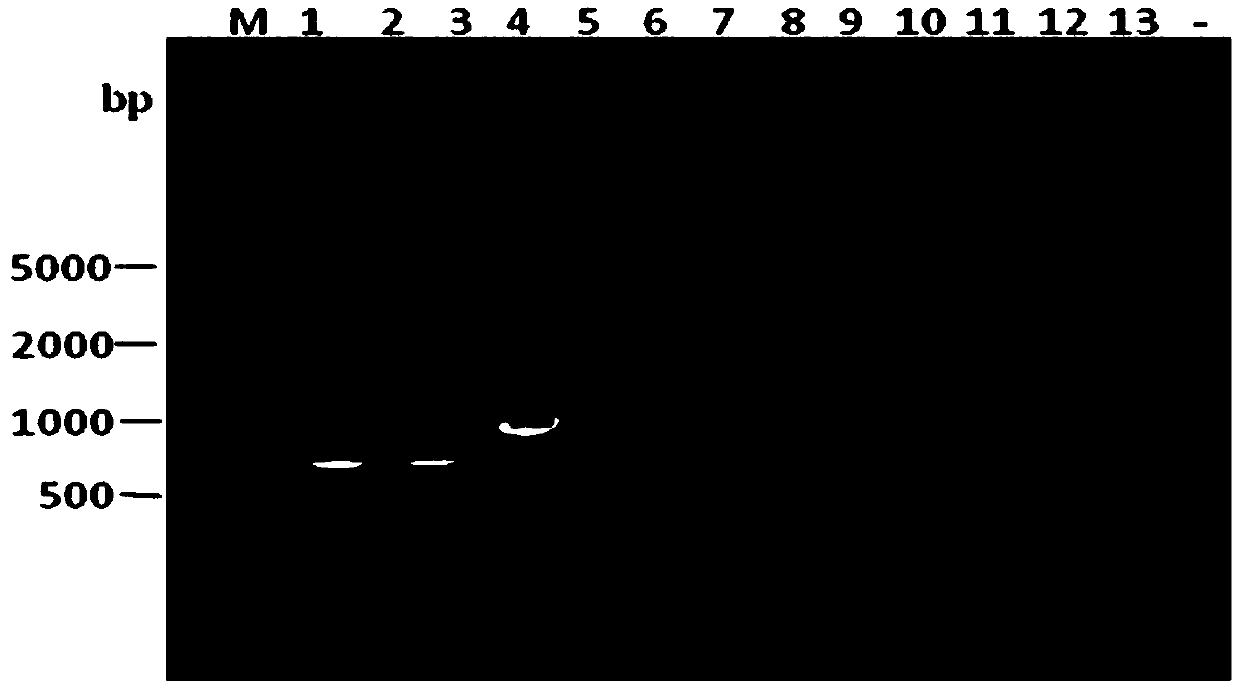
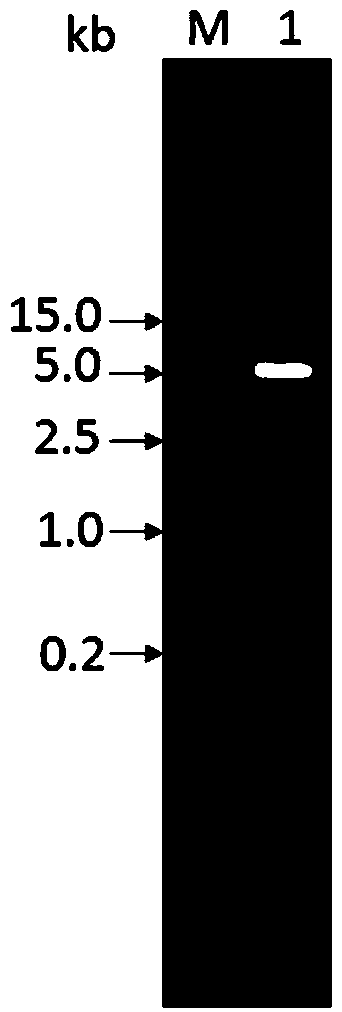
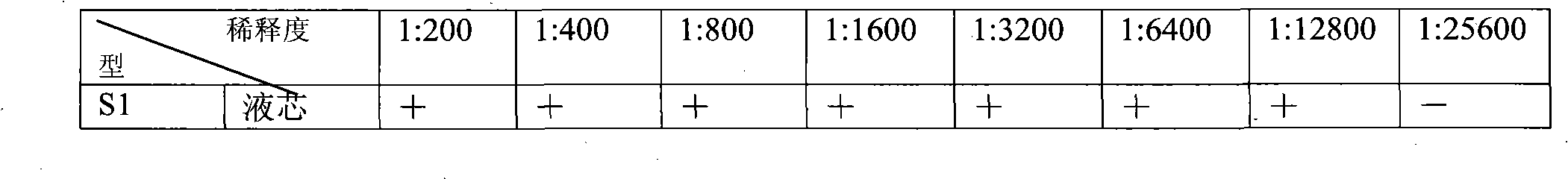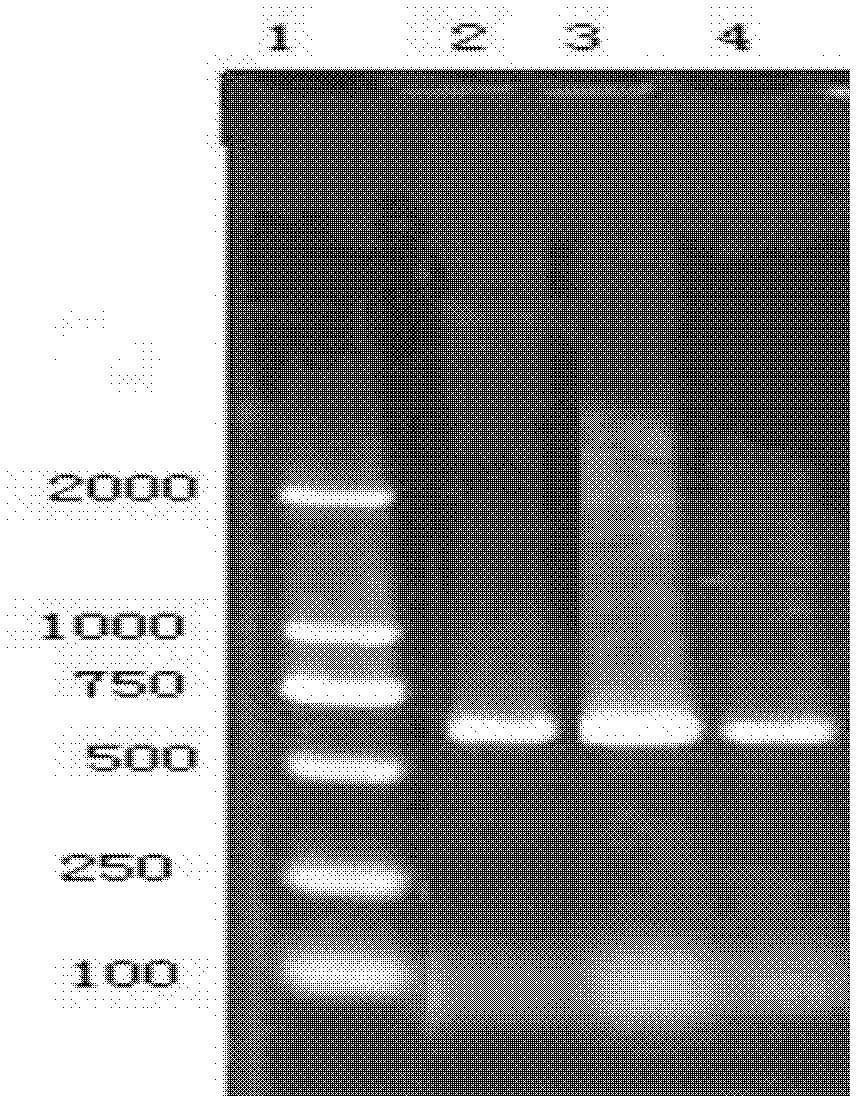Patents
Literature
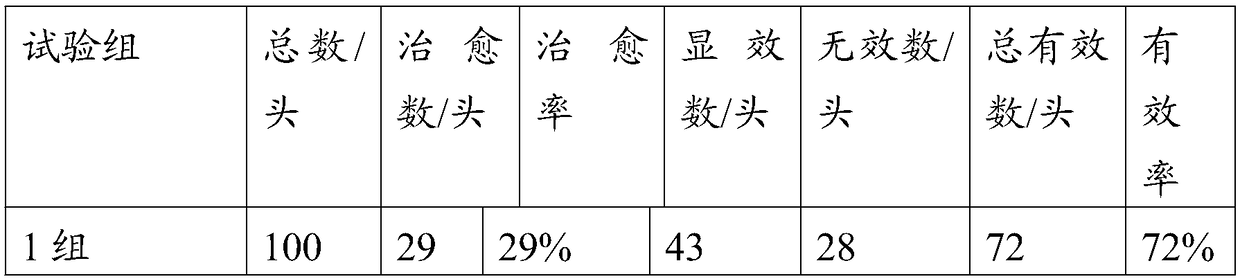
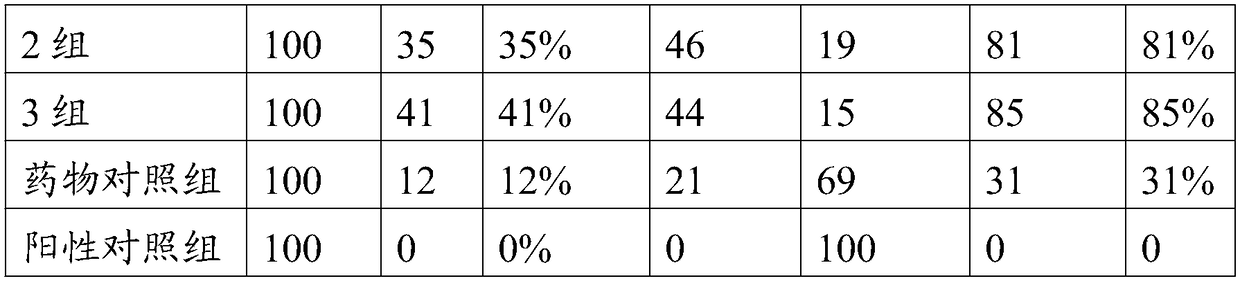
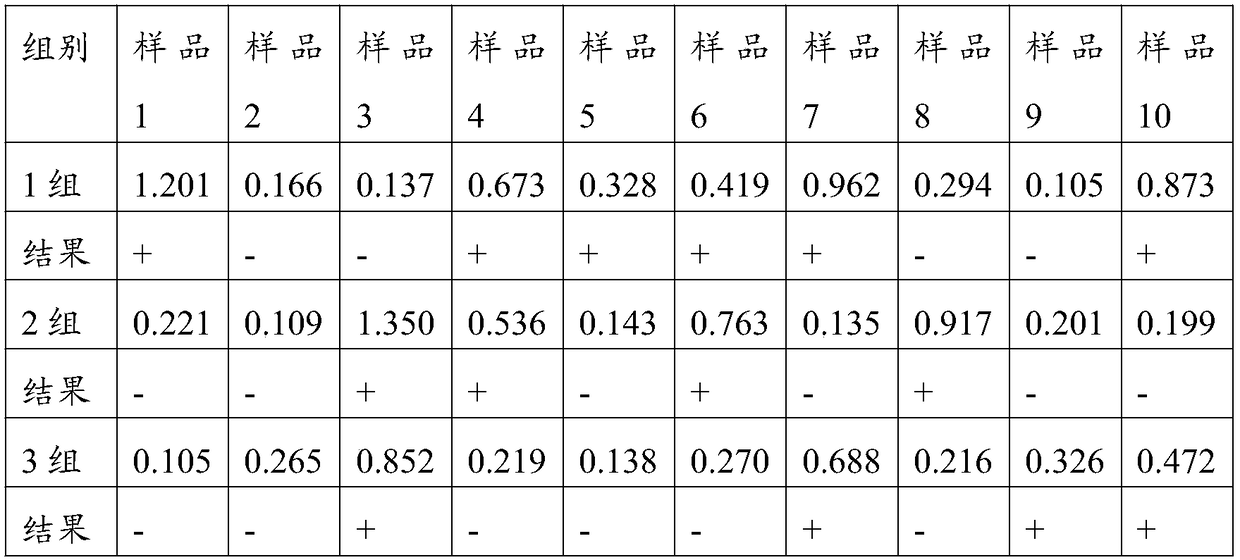
40 results about "Pleuronectes pinnifasciatus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colloidal gold chemiluminescence immune analysis method for detecting pig pleuropneumonia antibody
InactiveCN101113980ASuitable for testingHigh sensitivityChemiluminescene/bioluminescenceAnimal virusAntigen
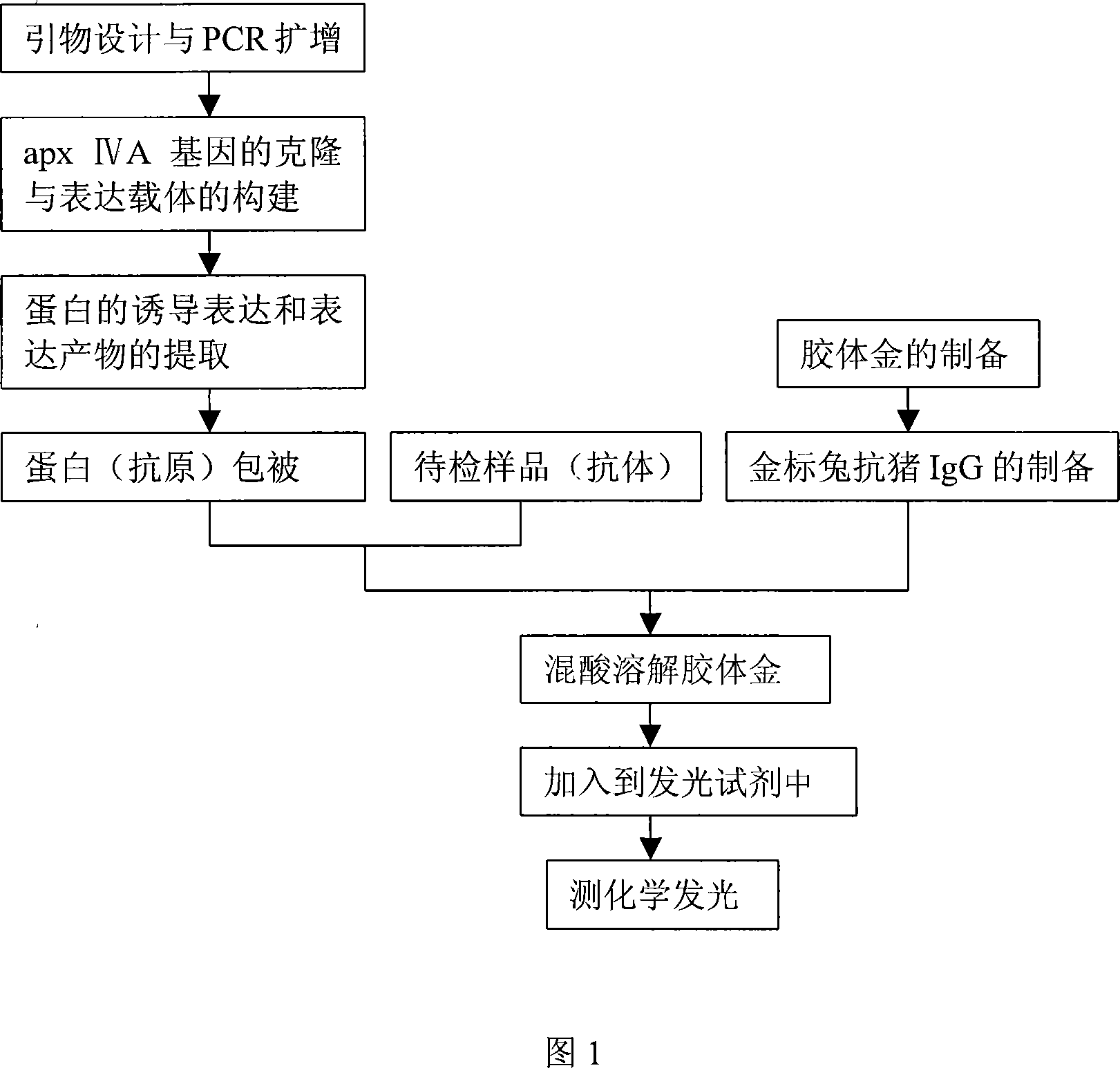
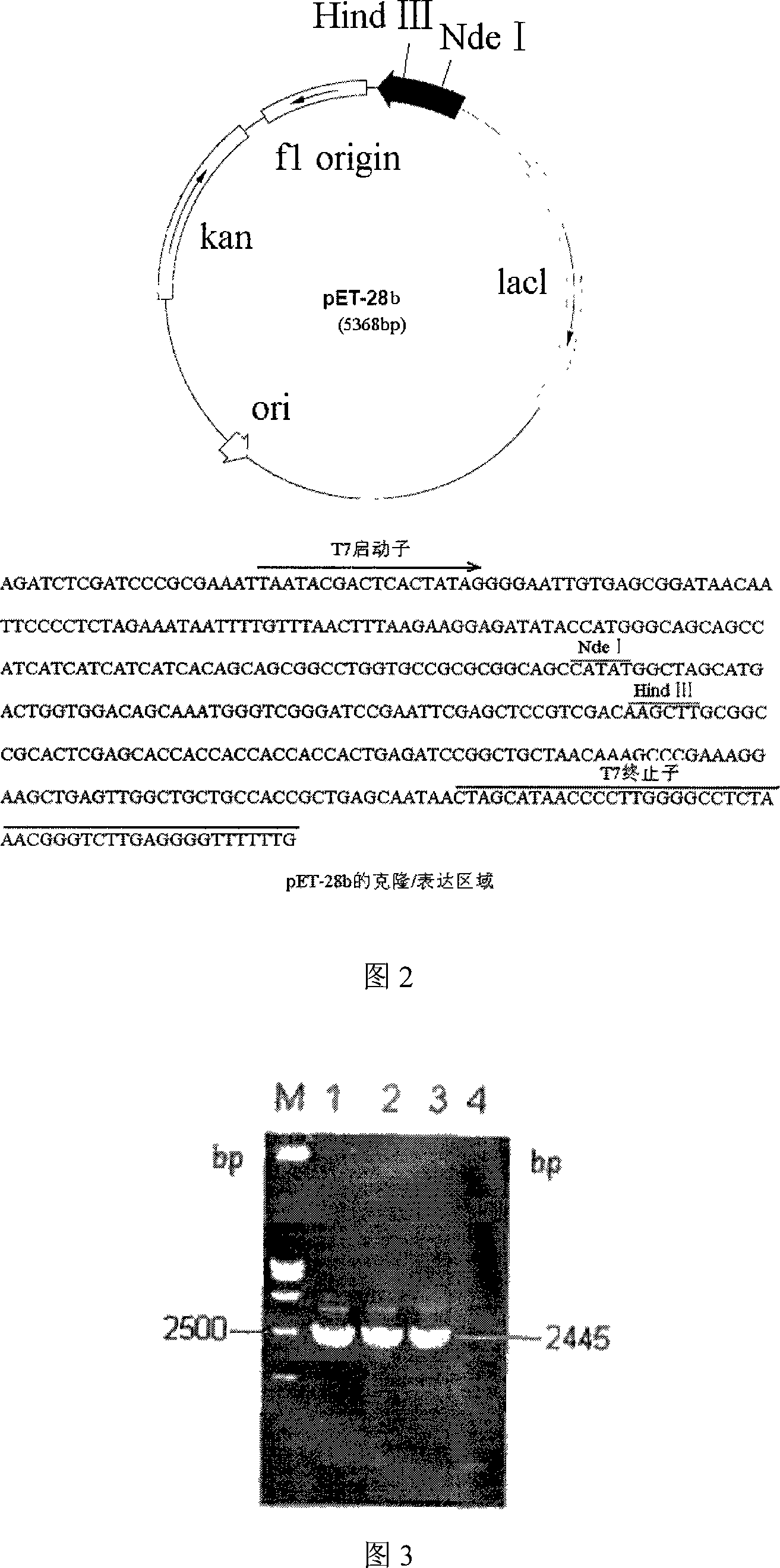
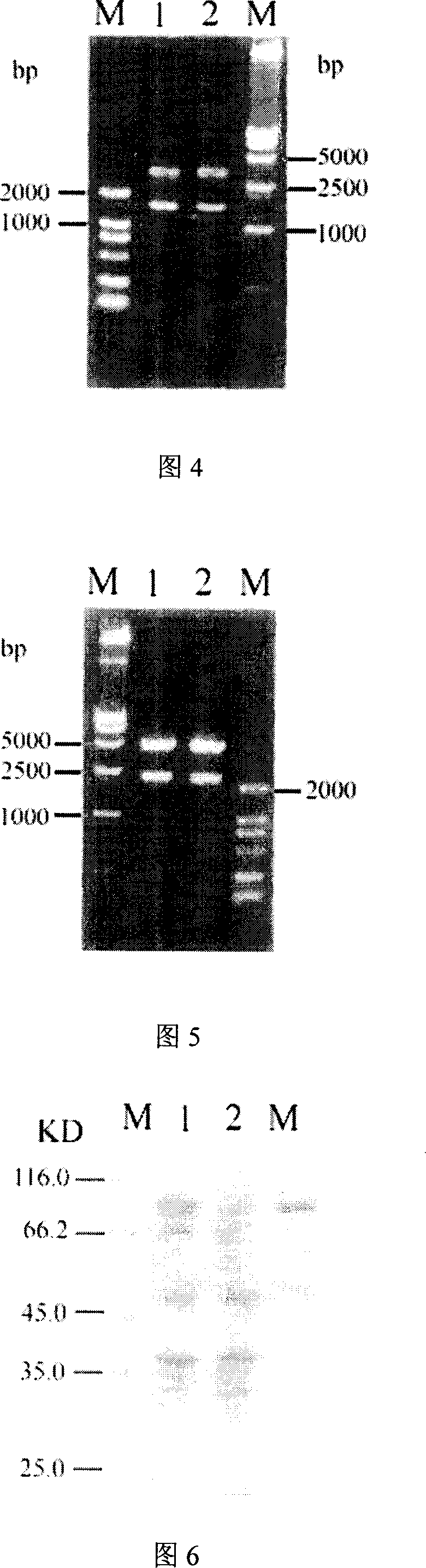
The invention discloses a colloidal gold chemiluminescence immune assay method of detecting pig peripneumonia antibody. The method relates to animal virus immunodetection and chemiluminescence immune assay field. The main points of the invention are hereinafter: fixing the pig peripneumonia Apx IVA antigen in 96-microtitor plate, blocking the spare active site in the plate, add sample product to be examined, thus the pig peripneumonia Apx IVA antigen is binded. The gold-labeled rabbit anti pig IgG is added and the specific reaction with the sample can take place. The chemiluminescence reagent is added with the dissolved solution after the colloidal gold labeled on rabbit anti pig IgG is dissolved by violet acid and then detect chemiluminescence intensity. The chemiluminescence intensity is in direct ratiot to antibody concentration to be detected, thus linear correlation is available between chemiluminescence intensity and pig peripneumonia Apx IVA antibody. Compared with the prior art, the method has more accuracy and high specificity to better meet the need of clinical detection of peripneumonia.
Owner:HUAZHONG AGRI UNIV
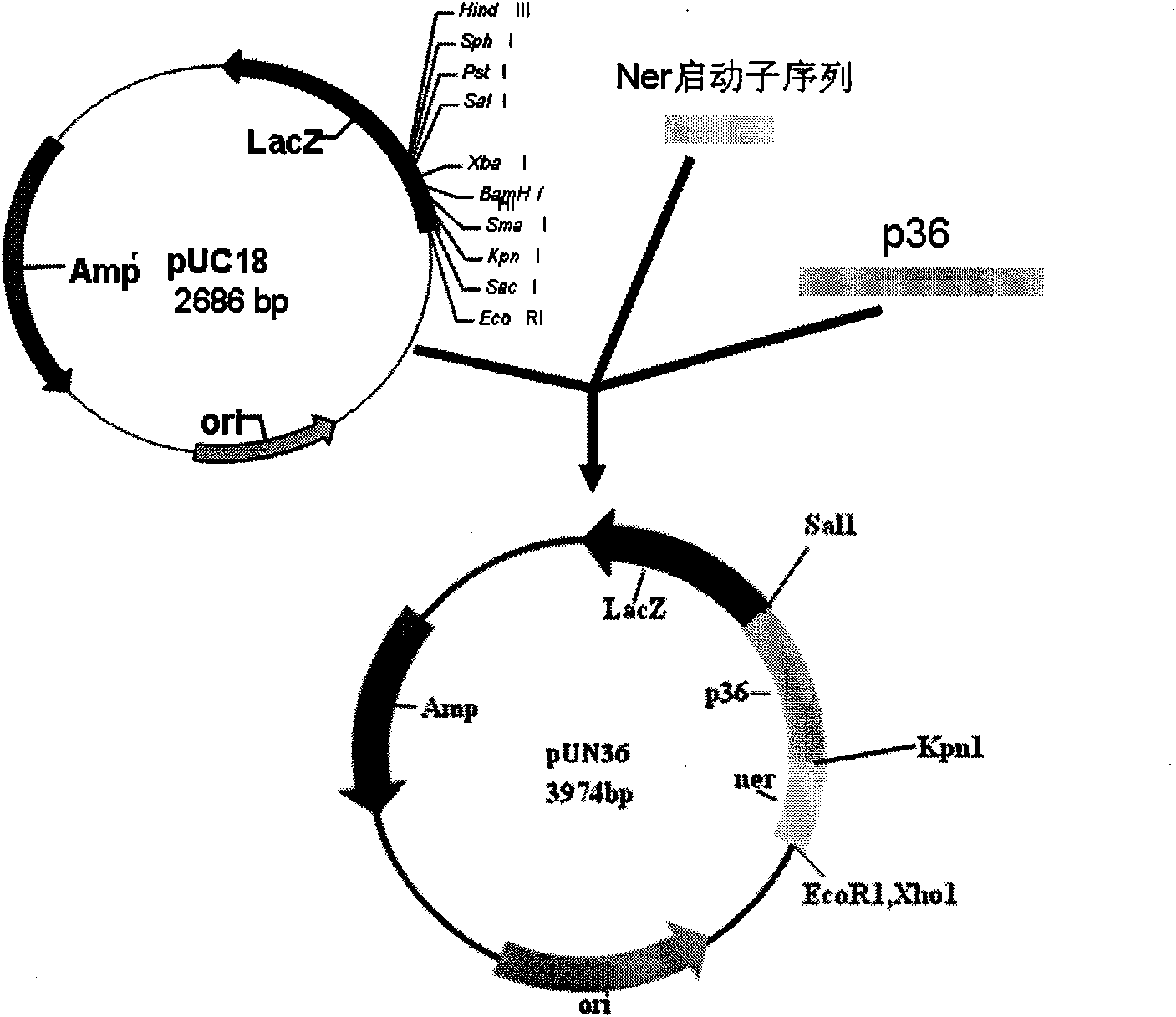
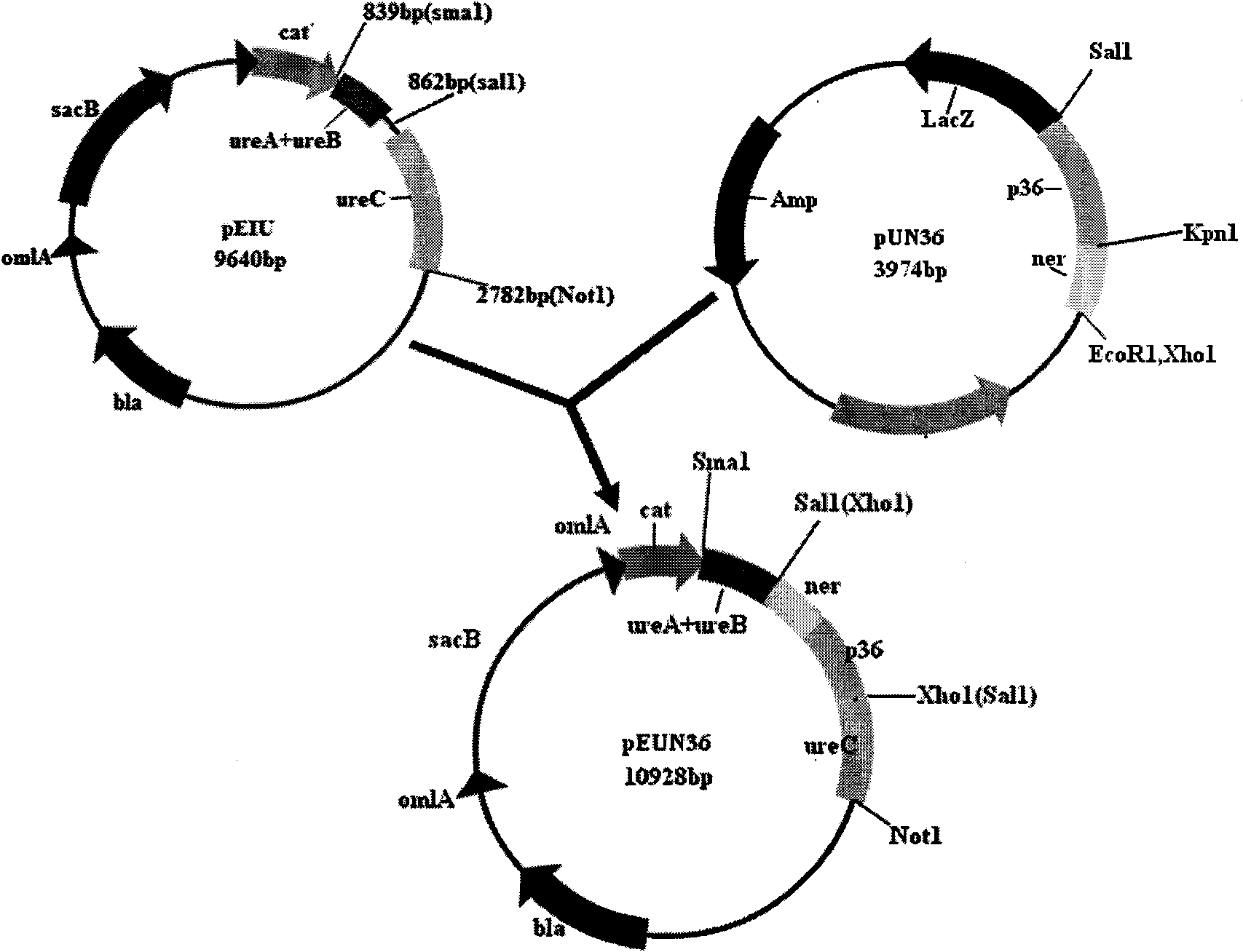
Porcine mycoplasmal pneumonia and porcine infectious actinobacillus pleuropneumoniae serum 1 type gene engineering strain vaccine and application thereof
InactiveCN101603024AEasy to buildStimulate systemic immunityAntibacterial agentsBacterial antigen ingredientsBacteroidesNucleotide
The invention belongs to the technical field of domestic animal infectious disease, relating to the technical field of bacterial gene engineering, in particular to a construction, vaccine preparation and application of a reconstruction porcine actinobacillus pleuropneumoniae serum 1 type and porcine mycoplasmal pneumonia double-gene engineering strain. The invention is characterized in that a strain of reconstruction porcine actinobacillus pleuropneumoniae serum 1 type low virulent strain SLW05, the reconstruction strain is preserved in China Center for Type Culture Collection, the preservation number is CCTCC NO: M209068, porcine mycoplasmal pneumonia antigenicity P36 gene segment is inserted into the chromosome of porcine actinobacillus pleuropneumoniae serum 1 type low virulent strain SLW03, and the nucleotide sequence of the P36 gene segment is shown in sequence table SEQ ID NO: 1. the invention also discloses a bivalent gene engineering vaccine prepared by using the reconstruction strain and an application thereof.
Owner:HUAZHONG AGRI UNIV
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
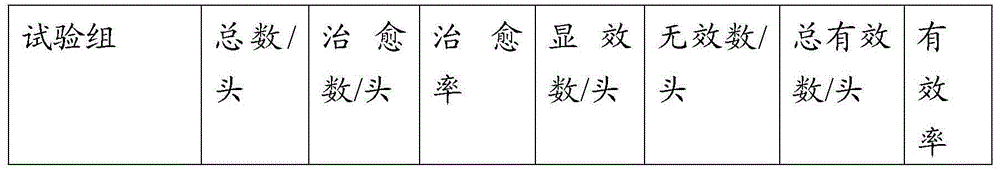
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
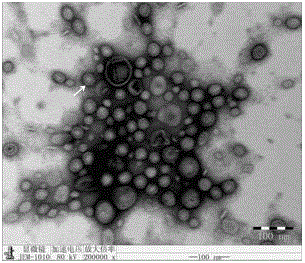
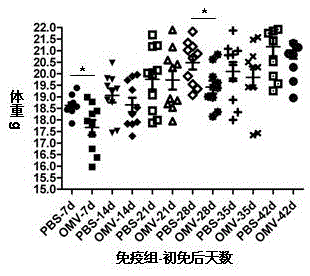
Preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and vaccine of APP OMVs
ActiveCN105420161AExperimental evaluation of immunostimulatory effectsAssessing immunostimulatory effectsAntibacterial agentsBacterial antigen ingredientsIMMUNE STIMULANTSFiltration
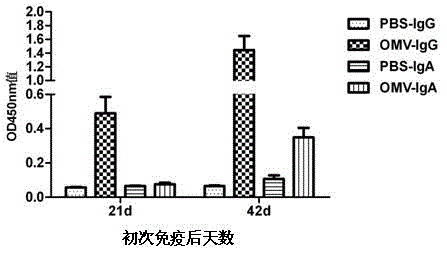
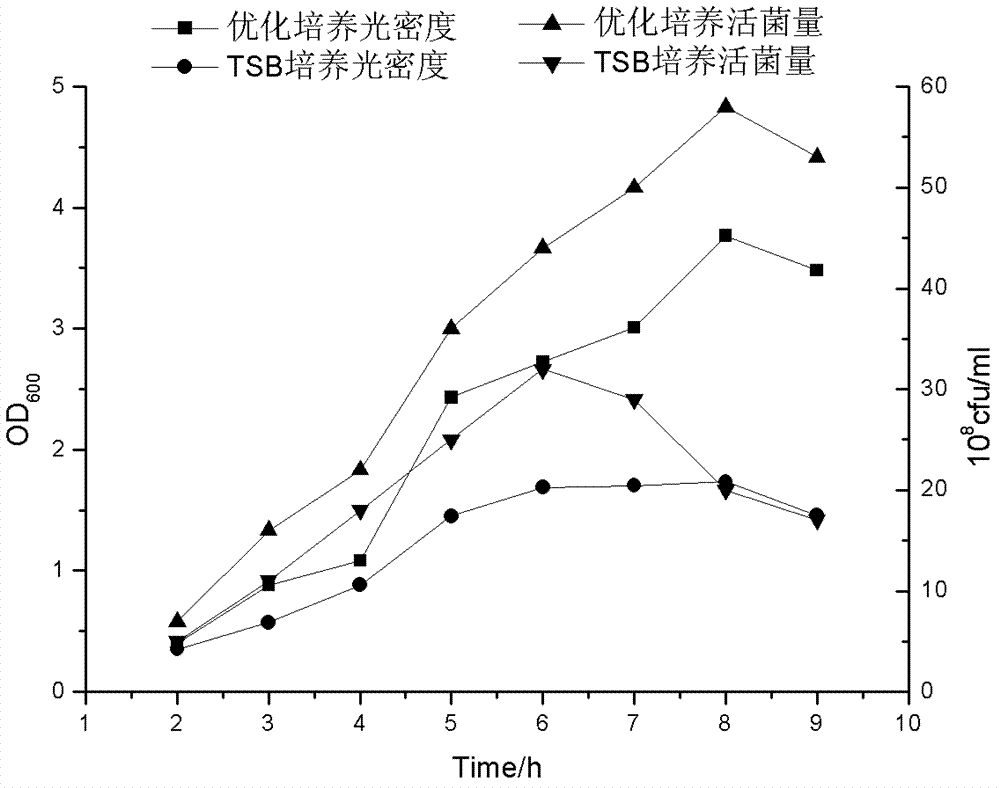
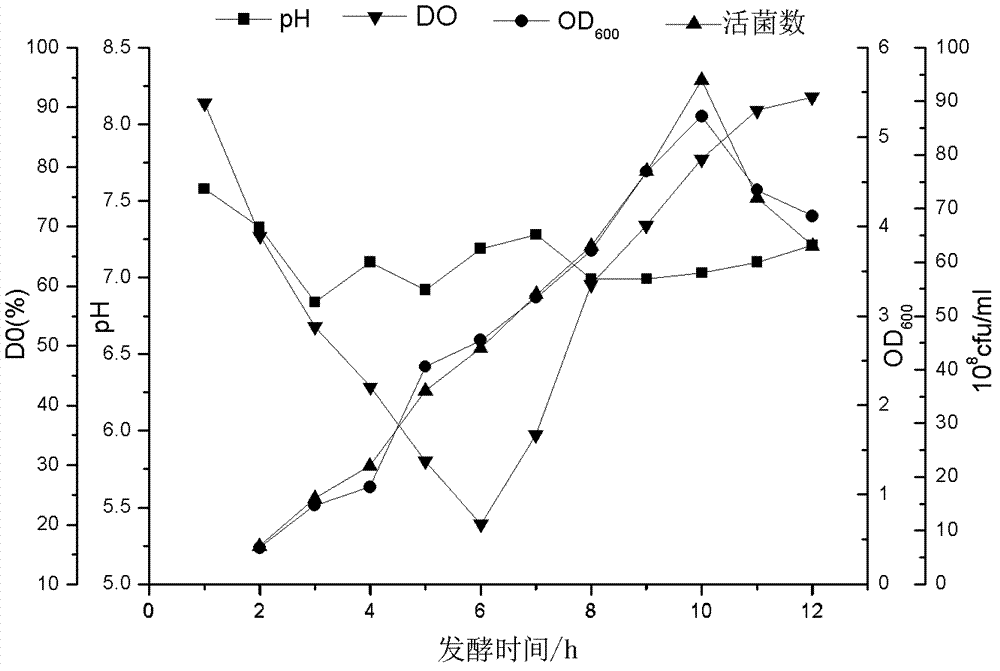
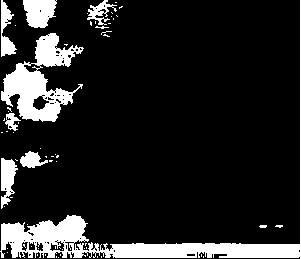
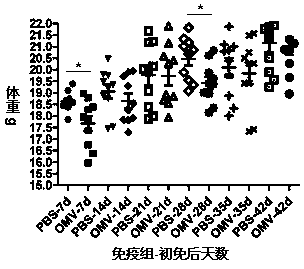
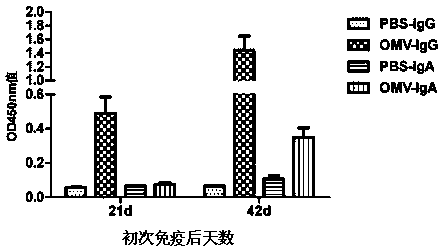
The invention relates to a preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and a vaccine of the APP OMVs, and belongs to the technical field of biology. The preparation method comprises the steps of culturing APP shope strains in vitro by using a culture medium which is in iron ion limit, obtaining acellular cultural supernatant after carrying out centrifugation and 0.22-[mu]m filtration treatment, and preparing the OMVs released by germs through ultracentrifugation, wherein the observation through a transmission electron microscope shows that the diameter of most OMVs is 50 to 100 nm, the OMVs are used as subunit vaccines to carry out secondary intranasal immunization on a mouse, the weight of the OMV immune mouse is increased for a long time, and the visual forms of lungs have no obvious difference from a PBS (Phosphate Buffer Saline) immune group. Meanwhile, an experiment shows that the OMVs are efficient immune stimulants, not only can high-level IgG (Immunoglobulin G) be stimulated to be generated by mouse sera, but also high-level IgA (Immunoglobulin A) can be generated in mouse lungs, mucosal immunity of the mouse lungs is effectively stimulated, and a better application prospect of using the OMVs as the subunit vaccines is expressed.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Actinobacillus pleuropneumoniae serotype 2 bacterial strain and its preparation method
ActiveCN102391975AHigh purityHigh immune protection against virusesBacteriaMicroorganism based processesMonosodium glutamateAntigen
The invention discloses an actinobacillus pleuropneumoniae serotype 2 bacterial strain and its preparation method, which is actinobacillus pleuropneumoniae serotype 2XT, wherein the CCTCC NO. is M2011410. The preparation method comprises the following steps: 1) medium preparation, A) weighting yeast powder, glucose, monosodium glutamate, MgSO4, FeSO4.7H2O, dissolving into pure water, regulating pH value and disinfecting; B) weighting NaH2PO4 and K2HPO4 to prepare mother liquor and disinfecting; C) weighting NAD to prepare mother liquor, filtering by a filter membrane; D) mixing the solutions obtained in the step A, the step B and the step C according to amount to obtain the medium; 2) fermentation and culture, a) activating the freeze-drying seeds by NAD-contained TSA plates until single colony is grown out; b) selecting single colony and culturing by shaking a bottle; c) transferring cultured seed liquid to the fermentation medium; d) low stirring at initial fermentation period, and low ventilating; e) raising rotating speed at logarithmic phase and ventilating, and on-line controlling pH value; f) improving dissolved oxygen, and placing into a tank and collecting bacterium. The actinobacillus pleuropneumoniae serotype 2 bacterial strain suitable for preparing inactivated vaccine has the advantages of strong toxicity, good antigen effect, low price, fast mycelium growth, highdensity and easy control.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Immunoprotective antigen protein APJL_1380 for actinobacillus pleuropneumoniae and application thereof
ActiveCN108794584AImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsProtective antigenActinobacillus pleuropneumoniae antibody
The invention discloses immunoprotective antigen protein APJL_1380 for actinobacillus pleuropneumoniae and application thereof. An amino acid sequence of the immunoprotective antigen protein for the actinobacillus pleuropneumoniae is shown in SEQ ID NO.1. The immunoprotective antigen protein consists of 273 amino acids, and the matured polypeptide part has amino acids at the 25th to 582nd sites; anucleotide sequence for coding the immunoprotective antigen protein is shown in SEQ ID NO.2. The immunoprotective antigen protein has the advantages that the immunogenicity is good, and the immunoprotective function is strong; the immunoprotective antigen protein can be applied to preparation of kits for detecting actinobacillus pleuropneumoniae antibodies, preparation of swine pleuropneumoniae sub-unit vaccines, and preparation of medicines for preventing diseases caused by the actinobacillus pleuropneumoniae; a novel material is provided for the preparation of the swine pleuropneumoniae sub-unit vaccines, and the important meaning is realized in the preventing and treatment of the swine pleuropneumoniae.
Owner:HUAZHONG NORMAL UNIV
Porcine actinobacillus pleuropneumoniae attenuated strain and porcine pleuropneumonia-preventing product prepared from same
InactiveCN104726387AReduced toxicityLow toxicityAntibacterial agentsBacterial antigen ingredientsBiotechnologyImmunogenicity
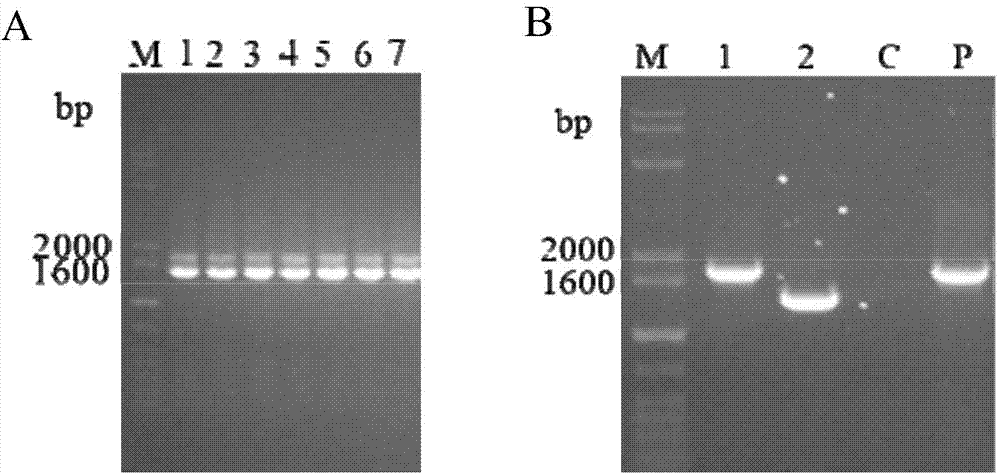
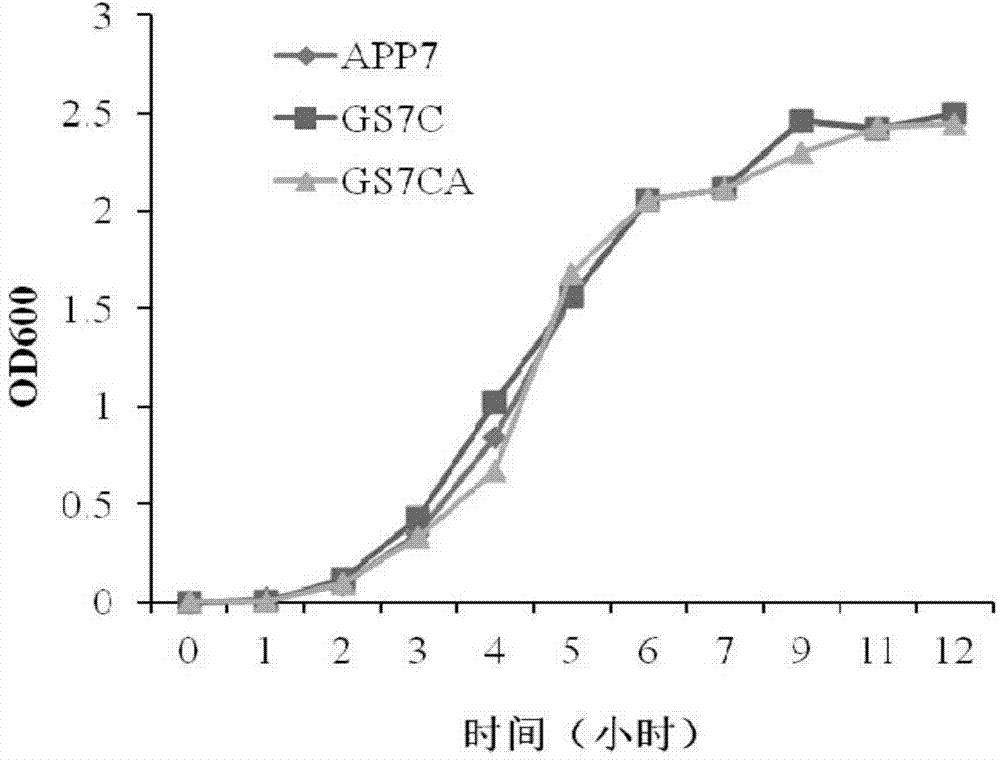
The invention discloses a porcine actinobacillus pleuropneumoniae attenuated strain and a porcine pleuropneumonia-preventing product prepared from the same, and relates to the fields of microorganisms and immunology. According to the porcine actinobacillus pleuropneumoniae attenuated strain and the porcine pleuropneumonia-preventing product prepared from the same disclosed by the invention, on the basis of a single mutant strain GS7C, a ureC gene segment in a genome is further deleted, and an apxIII-N gene segment with immunogenicity is inserted, and then the double mutant strains (GS7CA) of apxIIC- / apxIA+ and ureC- / apxIII+ which successfully express ApxIII-N proteins are screened by virtue of a sacB negative gene screening system, and the collection number is CGMCC NO. 10016. Experimental data indicates that, the hemolytic activity and urease activity of the double mutant strains are completely lost, the toxicity is greatly reduced, and stable inheritance can be realized; the attenuated live vaccine prepared by the porcine actinobacillus pleuropneumoniae attenuated strain disclosed by the invention has low toxicity, cross protection activity, high bio-safety and stable quality.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Vaccine of swine fever-porcine contagious pleuropneumoniae bivalent subunit and preparation method and applications thereof
ActiveCN110038124AAvoid infectionNo cross interferenceAntibacterial agentsSsRNA viruses positive-senseSerum igeClassical swine fever virus E2
The invention relates to the technical field of veterinary drugs, in particular to a vaccine of swine fever-porcine contagious pleuropneumoniae bivalent subunit and a preparation method and applications thereof. The vaccine comprises classical swine fever virus E2 protein, serum type-7 actinobacillus pleuropneumoniae toxin protein ApxII, serum type-1 actinobacillus pleuropneumoniae outer membraneprotein Oml and vaccine adjuvant. The vaccine is obtained by mixing and emulsifying the proteins and the vaccine adjuvant in equal volume. The vaccine can simultaneously prevent swine fever and porcine contagious pleuropneumoniae caused by serum type-1 and type-7 porcine actinobacillus pleuropneumoniae, and has good effect and low cost.
Owner:天康生物制药有限公司
Reagent box for inspecting infection of swine pleuropneumonia actinomyces
InactiveCN1645148AGood antigenicityEasy to buildSugar derivativesBiological testingAntigenPeroxidase
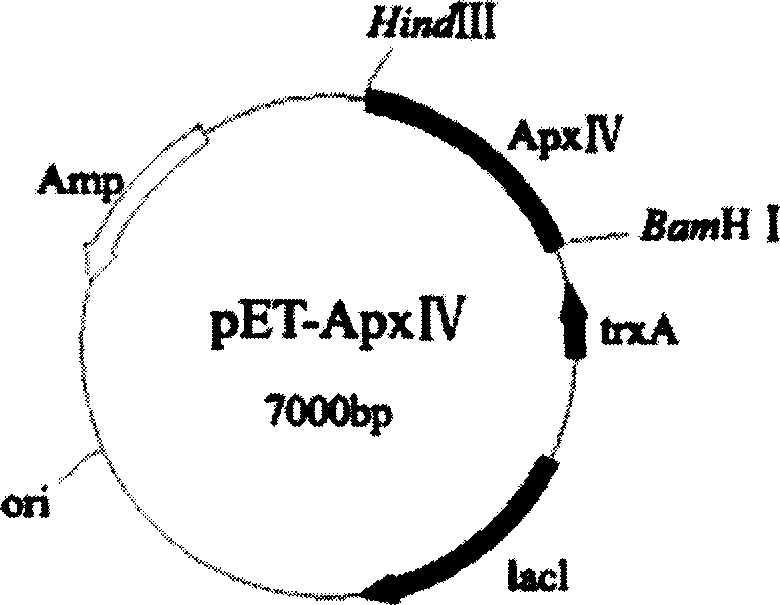
A reagent kit consists of enzyme labeled board, IgG antibody, citric acid sodium bicarbonate, colour display system, positive and negative serum. It is featured as using PCR to amplify target gene of ApxIV gene, forming expression plasmid PET32a-ApexIV containing target gene, converting plasmid to be host bacterium BL21(DE3) and purifying in vitro expression protein to be antigen, setting up ELISA detection method and optimizing reaction condition to form the reagent kit.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
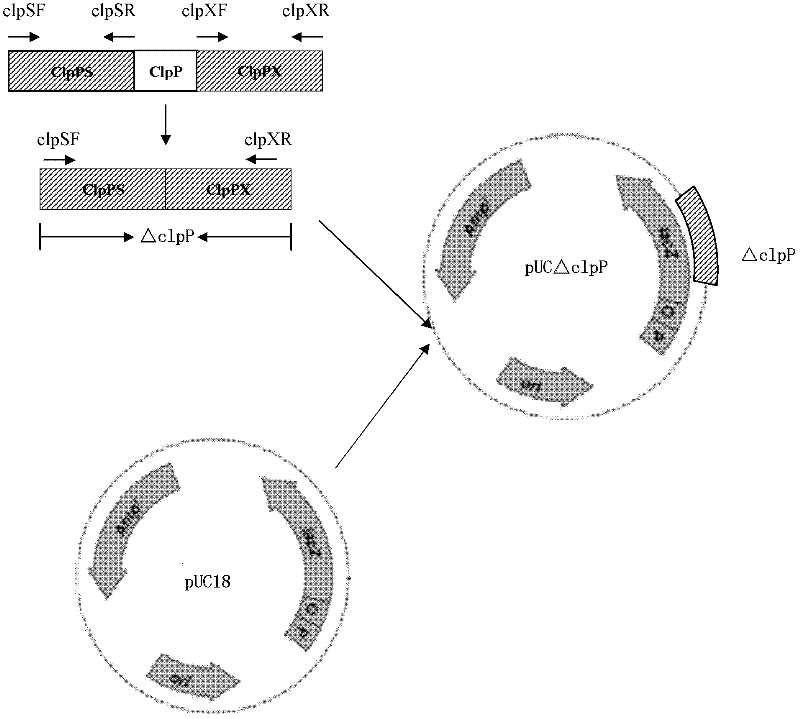
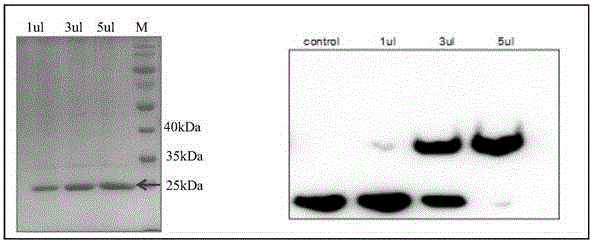
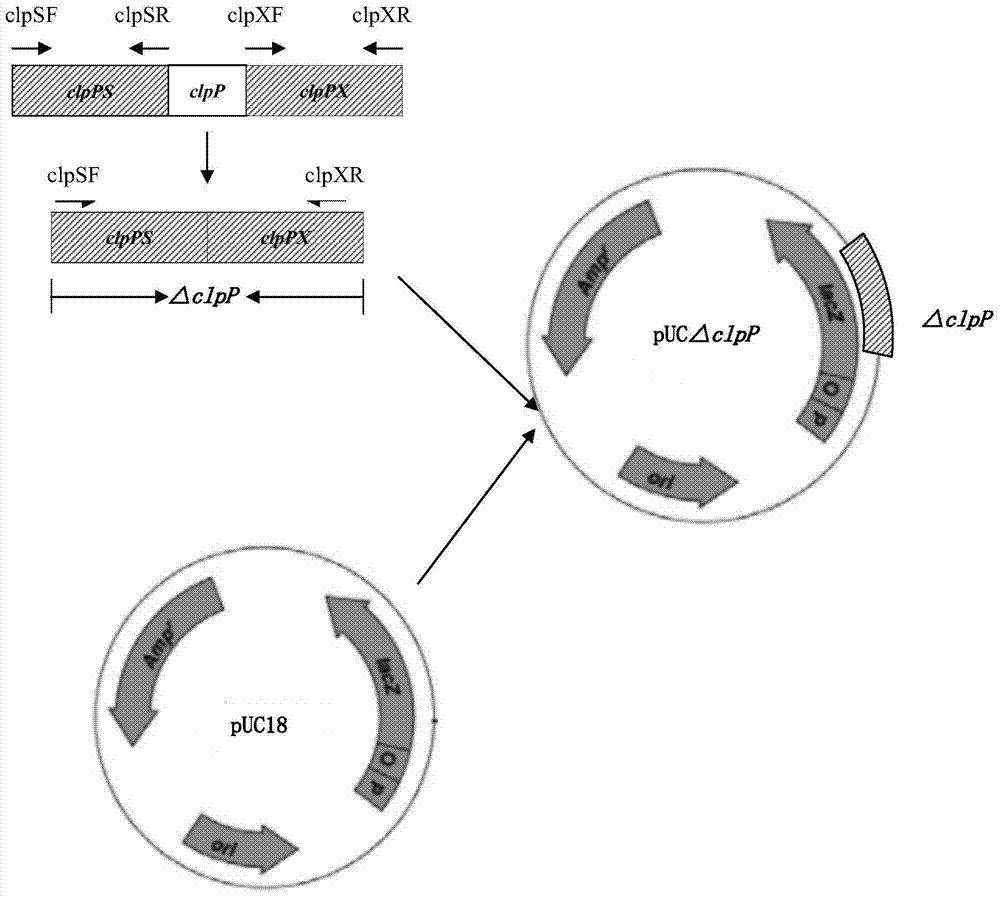
Porcine actinobacillus pleuropeumoniae ClpP protease gene-deleted strain containing no resistance marker, construction method thereof, and application thereof
InactiveCN102443552AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyGenetic engineering
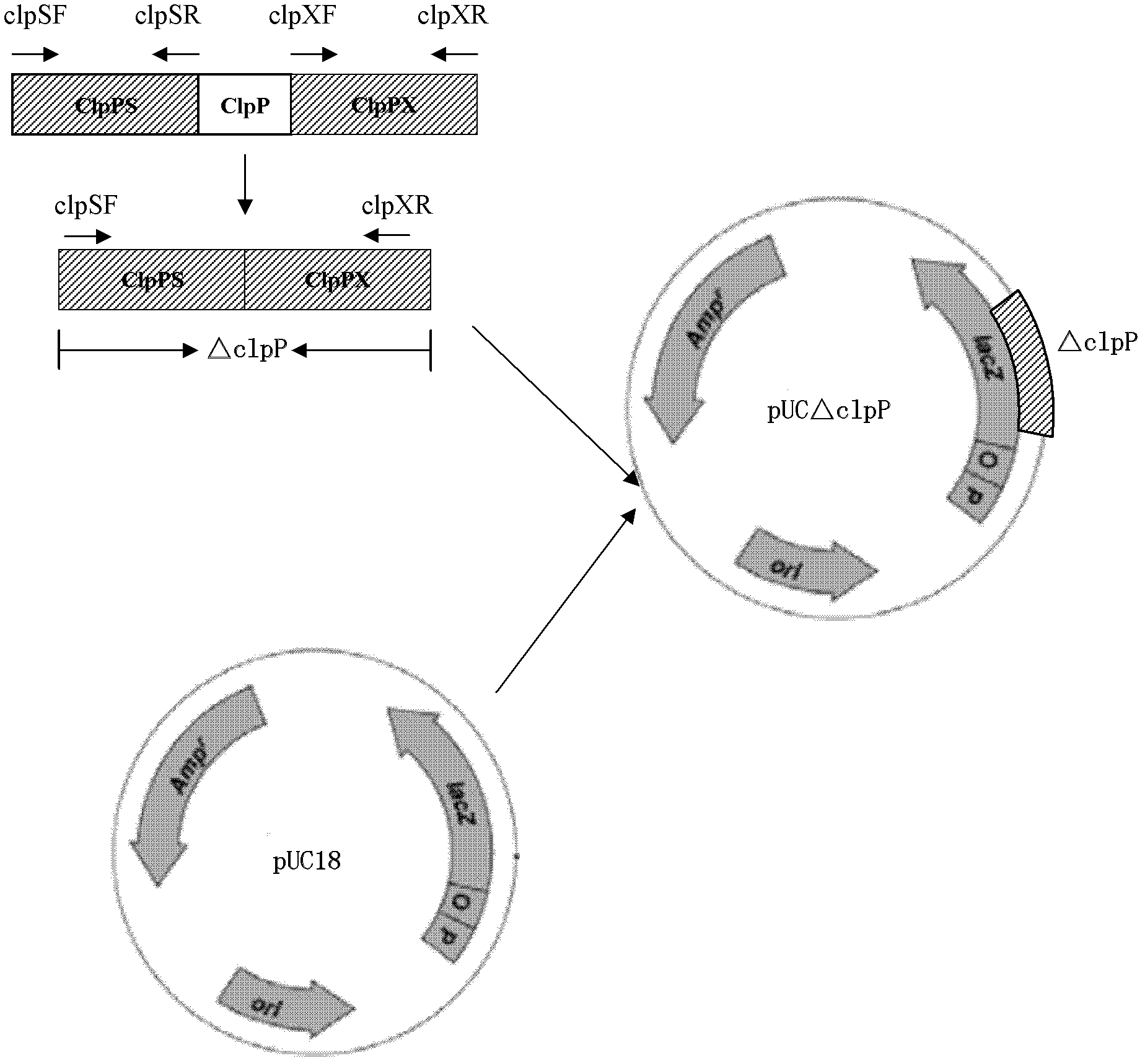
The invention discloses a porcine actinobacillus pleuropeumoniae ClpP protease gene-deleted strain containing no resistance marker, and a construction method thereof. The invention belongs to the technical field of bacteria genetic engineering. According to the invention, a recombinant strain APPdeltaclpP of actinobacillus pleuropeumoniae is a strain obtained through coding gene deactivation upon ClpP protease in actinobacillus pleuropeumoniae with an oriented homologous recombination technology, and the expression of the ClpP protease is damaged. With the technology provided by the invention, the virulence of the mutant strain is lower than a parent strain, and the mutant strain is safe to animals. Therefore, an important basis is provided for the modification upon porcine contagious pleuropneumonia vaccines and the development of corresponding differential diagnosis reagents. The strain and the method provided by the invention have important significances in promoting the eradication and purification of porcine contagious pleuropneumonia all around the world.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit of actinobacillus pleuropneumoniae and use thereof
InactiveCN100567504CStrong specificityHigh sensitivityMicrobiological testing/measurementActinobacillus pleuropneumoniaePositive control
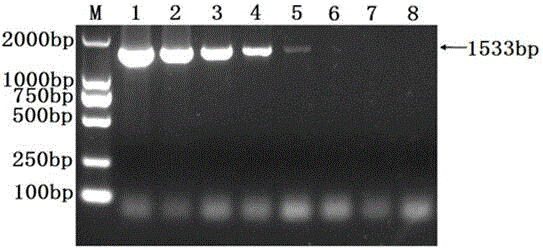
The invention discloses a detection kit for Actinobacillus pleuropneumoniae and its application. The kit provided by the present invention includes three pairs of primers, namely an inner primer pair, an outer primer pair and a circular primer pair combined with the 3' end 1000bp gene in the Actinobacillus pleuropneumoniae Gen Bank Accession Number AF021919 sequence. The kit also includes a loop-mediated isothermal amplification reagent, a positive control, a negative control and a fluorescent chromogenic reagent, and the positive control is the DNA of Actinobacillus pleuropneumoniae. The invention also provides a method for detecting whether the animal carries the Actinobacillus pleuropneumoniae by using the kit of the invention. The kit of the present invention has high detection sensitivity, 6-10 copies can detect the target DNA, is simple and convenient to operate, and is especially suitable for the detection of pathogens carried out at the grassroots level and the detection of Actinobacillus pleuropneumoniae in possibly contaminated animal food .
Owner:CHINA AGRI UNIV
Actinobacillus pleuropneumoniae immune protective antigen protein APJL_0922 and application thereof
ActiveCN108840913AImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsDiseaseProtective antigen
The invention discloses actinobacillus pleuropneumoniae immune protective antigen protein APJL_0922 and application thereof. The amino acid sequence of the actinobacillus pleuropneumoniae immune protective antigen protein is as shown in SEQ ID NO.1; and the immune protective antigen protein consists of 273 pieces of amino acid, and the mature polypeptide part is the 20th to the 273rd amino acid. The nucleotide sequence for encoding the immune protective antigen protein is preferably as shown in SEQ ID NO.2. The immune protective antigen protein has high immunogenicity and strong immunoprotection effect, and can be applied to preparation of a kit for detecting an actinobacillus pleuropneumoniae antibody, preparation of pig pleuropneumonia subunit vaccine and preparation of medicines for preventing diseases caused by the actinobacillus pleuropneumoniae. The invention provides a new material for preparing the pig pleuropneumonia subunit vaccine, and important significance in preventing and treating pig pleuropneumonia is achieved.
Owner:HUAZHONG NORMAL UNIV
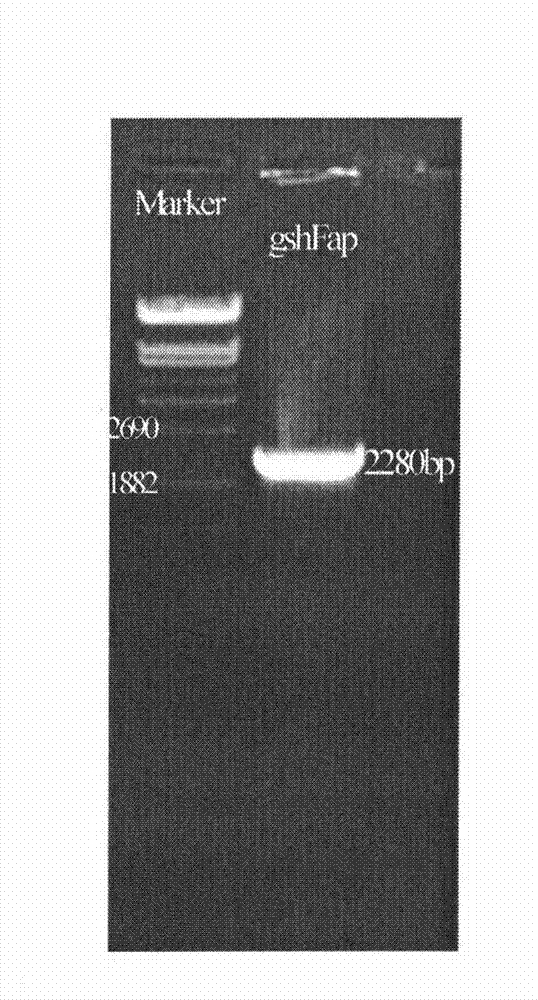
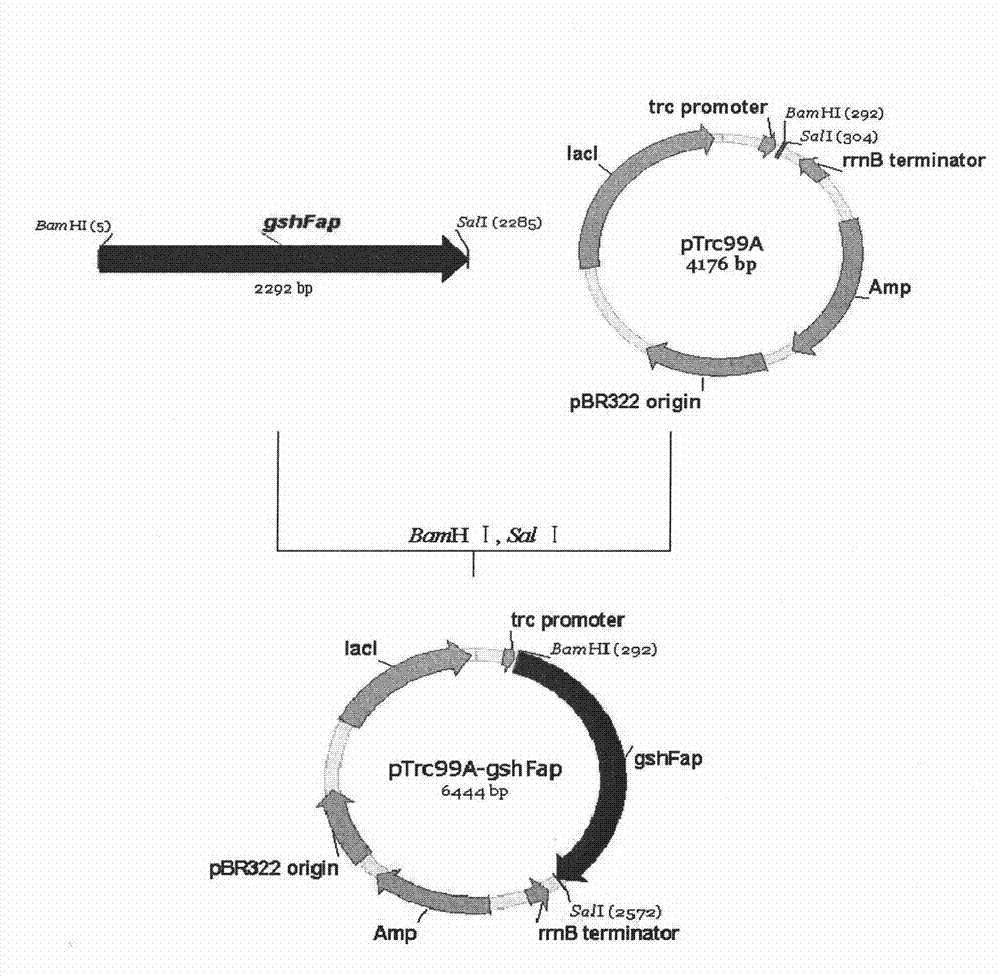
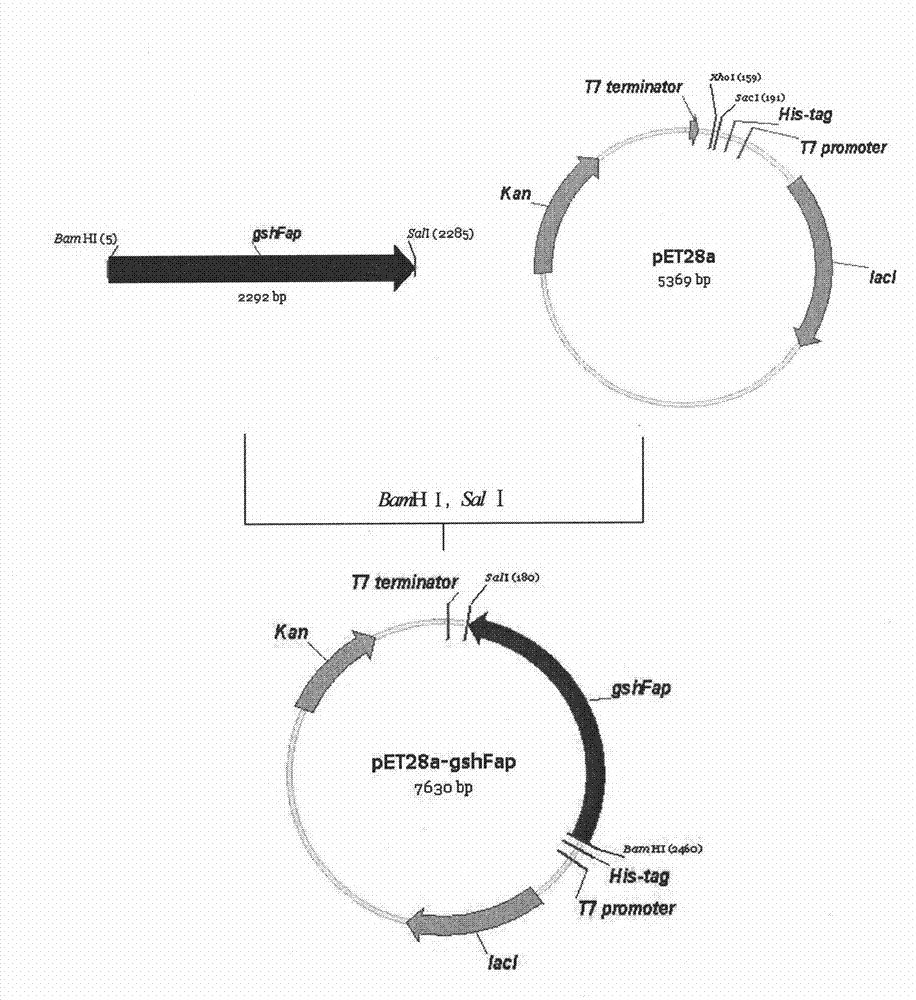
Bifunctional glutathione synthetase and method for producing glutathione by using same
InactiveCN102071171BIncrease production levelsMicroorganism based processesEnzymesHeterologousStreptococcus sanguinis
The invention discloses a method for producing glutathione by using bifunctional glutathione synthetase contained in actinobacillus pleuropneumonia, actinobacillus succinogenes, bacillus cereus, streptococcus sanguis, streptococcus gordonii, streptococcus uberis and streptococcus thermophilus. The method comprises heterologous expression by using the seven microbes or enzymes of the microbes in other microbes. The glutathione synthesized by using the method has the structure of natural glutathione, and has high response rate and high output and yield of the glutathione.
Owner:EAST CHINA UNIV OF SCI & TECH
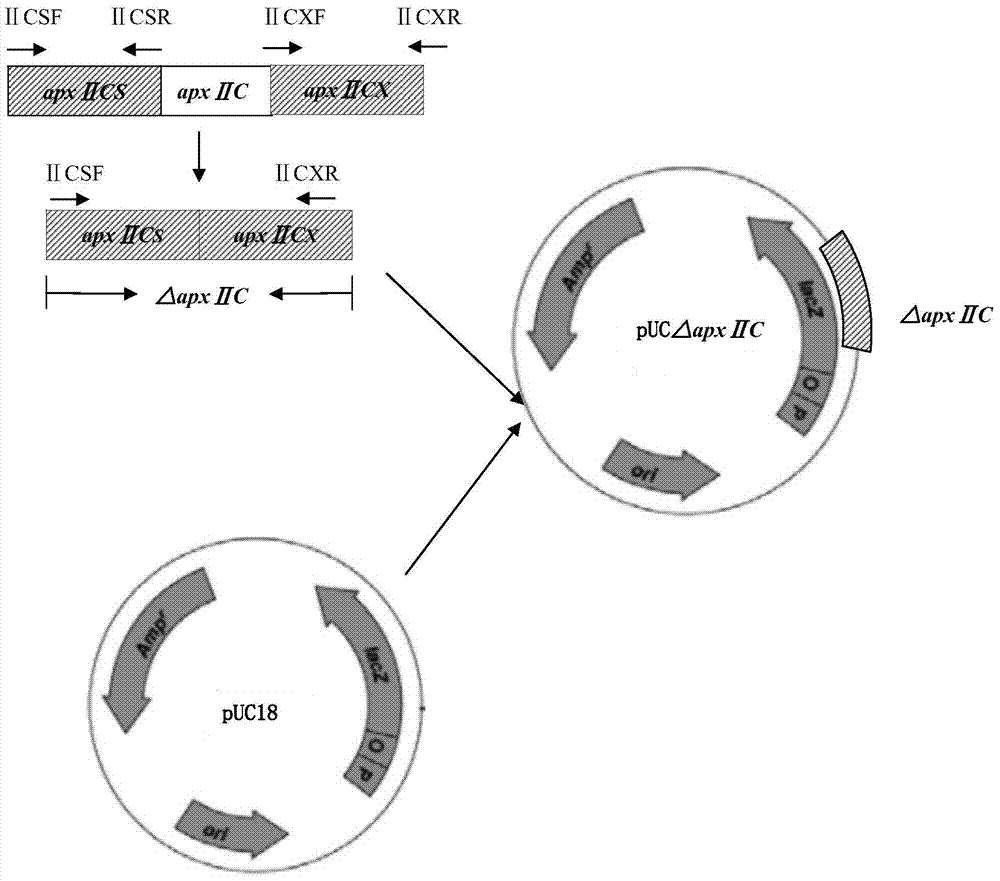
Resistance marker-free porcine actinobacillus pleuropneumoniae double-gene defective strain, construction method and application thereof
InactiveCN102517232AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyDifferential diagnosis
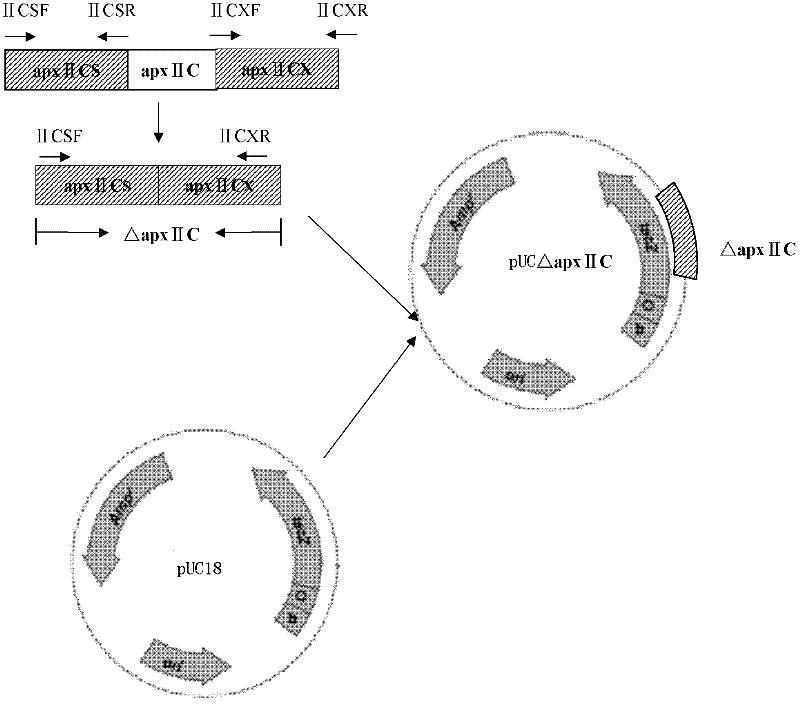
The invention discloses a resistance marker-free porcine actinobacillus pleuropneumoniae (APP) serum type 7 double-gene defective strain, and belongs to the technical field of bacterial gene engineering. The recombinant strain APPdeltaclpPdeltaapx II C of APP is obtained by inactivating ClpP protease in the APP and a coded gene of a hemolysin activated factor Apx II C by adopting a directional homologous recombination technology, and expression of the ClpP protease and the Apx II C protein is destroyed. The obtained double-gene defective strain has lower toxicity compared with a parent strain, is safe to animals, provides an important basis for transformation of porcine contagious pleuropneumonia (PCP) vaccines and research of matched identification and diagnosis reagents, and has great significance for promoting elimination and purification of the global PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for united typing detection of porcine contagious pleuropneumonia antibody and kit
InactiveCN101858912AMaintain native conformationHigh sensitivityFluorescence/phosphorescenceStatistical analysisBOAR
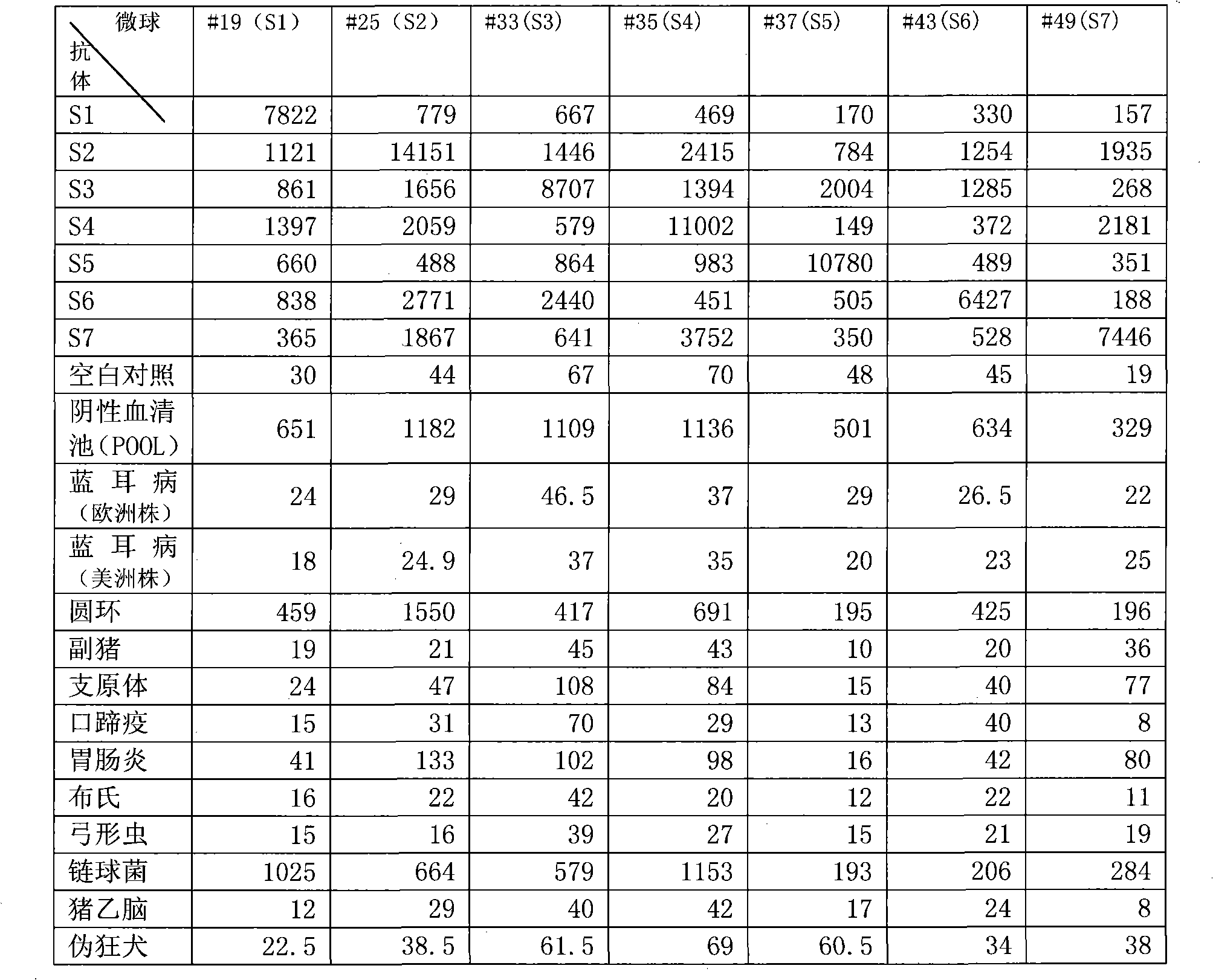
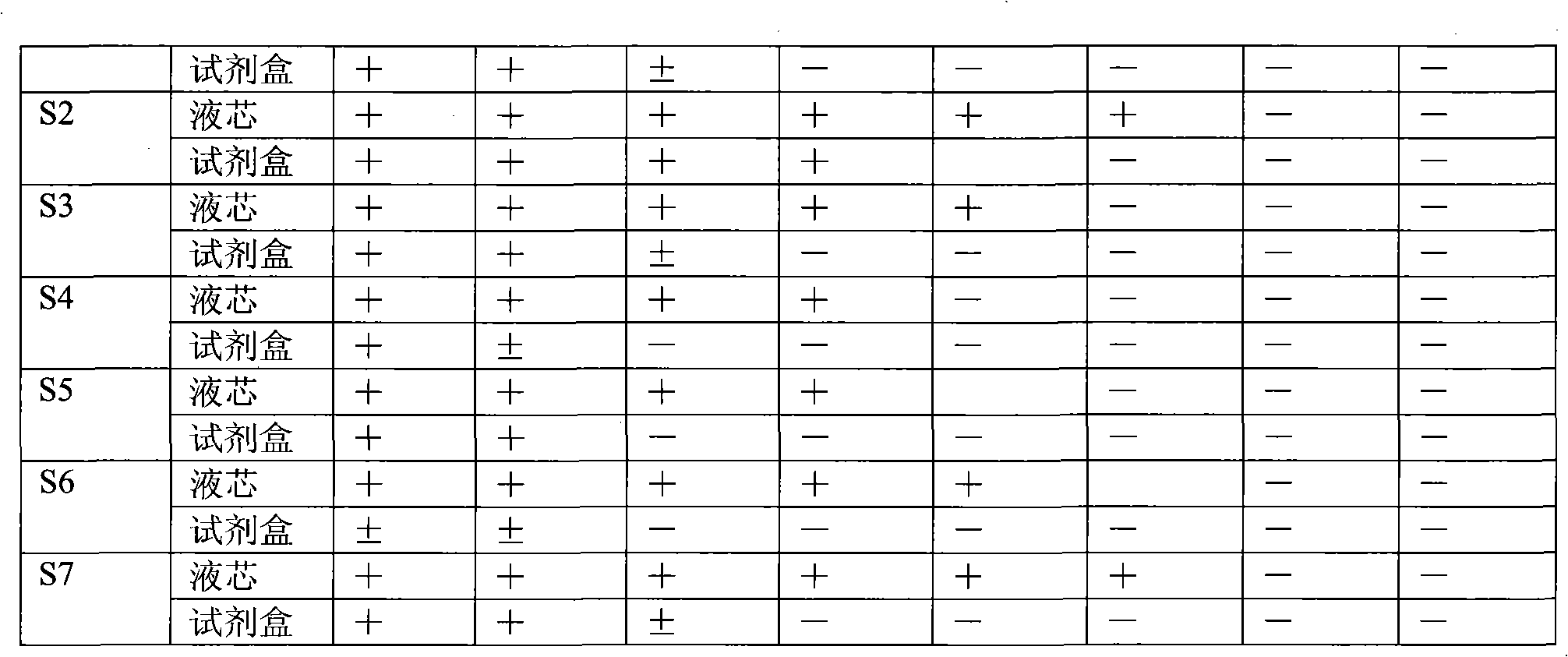
The invention discloses a method for united typing detection of a porcine contagious pleuropneumonia antibody. The method comprises the following steps: firstly preparing actinobacillus pleuropneumoniae polysaccharide antigen and rabbit anti-actinobacillus pleuropneumonia hyper-immune serum, and then purifying; coupling a liquid-phase chip microballoon by utilizing the purified rabbit anti-actinobacillus pleuropneumoniae hyper-immune serum, building a method for typing detection of the liquid-phase chip of the porcine pleuropneumonia antibody according to a double-sandwich ELISA principle, and determining the optimum experimental condition; and finally determining the threshold for positive and negative judgment of the liquid-phase chip through statistical analysis. In addition, the invention also discloses a kit for united typing detection of the liquid-phase chip of the porcine contagious pleuropneumoniae antibody. The invention can simultaneously carry out typing detection of the S1-S7-type serum antibody of porcine contagious pleuropneumonia, and the whole reaction can be completed within 3 hours; and the method has the characteristics of rapidly, sensitively, specifically andsimultaneously detecting a plurality of serum types, thus the method can be used for preliminarily screening entry and exit boars and diagnosing and monitoring porcine contagious pleuropneumoniae in hogpens of China.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Compound PCR typing kit for distinguishing serotypes of eight pig actinobacillus pleuropneumoniae and application thereof
ActiveCN108676900AImprove accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlNucleotide
The invention belongs to the technical field of biotechnology, and particularly relates to a composite polymerase chain reaction (PCR) typing kit for distinguishing serotypes of eight pig actinobacillus pleuropneumoniae and application thereof. The composite PCR typing kit comprises a PCR test tube, a PCR mixed enzyme, five pairs of specific primers, a positive control, a negative control and ddH2O, wherein the nucleotide sequences of the five pairs of specific primers are shown by SEQ ID NO:1-10. The composite PCR typing kit is used for distinguishing serotypes of eight pig pleuropneumonia actinobacillus of type 1, type 3, type 4, type 5, type 6, type 10, type 12 and type 14, and has high accuracy, good specificity and sensitivity up to a pg level. Sources of samples for identification include isolated strain resources or animal tissues, and a tissue washing liquid or a single colony and a bacterial solution can be directly cracked by boiling water as a template, thus eliminating tedious extraction of bacterial genomes and greatly saving time, manpower and cost.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Immunoprotective antigen protein APJL_1976 for Actinobacillus pleuropneumoniae and application of immunoprotective antigen protein APJL_1976
ActiveCN108822192AImproving immunogenicityImprove protectionAntibacterial agentsBacteriaProtective antigenActinobacillus pleuropneumoniae antibody
The invention discloses immunoprotective antigen protein APJL_1976 for Actinobacillus pleuropneumoniae and an application of the immunoprotective antigen protein APJL_1976. The amino acid sequence ofthe immunoprotective antigen protein for the Actinobacillus pleuropneumoniae is shown as SEQ ID NO.1, the immunoprotective antigen protein contains 273 amino acids, and a mature polypeptide part of the protein refers to 21-191-position amino acids. A nucleotide sequence for encoding the immunoprotective antigen protein is preferably shown as SEQ ID NO.2. The immunoprotective antigen protein has good immunogenicity and strong immunoprotective effect and can be applied to preparation of a kit for detecting an Actinobacillus pleuropneumoniae antibody, preparation of a subunit vaccine for porcinepleuropneumonia and preparation of a drug for preventing diseases caused by the Actinobacillus pleuropneumoniae. A new material is provided for preparing the subunit vaccine for porcine pleuropneumonia and has great significance in preventing and treating porcine pleuropneumonia.
Owner:HUAZHONG NORMAL UNIV
A detection kit for Haemophilus parasuis and its detection method
ActiveCN104711359BHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesStaphylococcus aureusPosition control
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and preparation method
ActiveCN105147802AEffective treatmentHas the effect of detoxifying and relieving asthmaAntibacterial agentsAnthropod material medical ingredientsVeterinary DrugsTherapeutic effect
The invention relates to the field of veterinary drugs, in particulrl to a composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and a preparation method thereof. The composition is prepared from, by weight, 15-20 parts of rhizoma atractylodis, 15-20 parts of radix saposhnikoviae, 10-13 parts of liquorice, 10-15 parts of apricot kernels, 30-35 parts of radix astragali, 15-20 parts of radix asteris, 10-15 parts of flos farfarae, 10-15 parts of herba ephedrae, 10-15 parts of periostracum cicada, 10-15 parts of fructus forsythiae and 20-30 parts of lonicera japonica. The composition has the effects of clearing heat, discharging fire, diminishing inflammation, resisting bacteria and the like, all the traditional Chinese medicines are proportioned reasonably to complement and coordinate mutually, the good treatment effect on the swine mycoplasmal pneumonia and the porcine contagious pleuropneumonia is achieved, the treatment course is short, the effects are rapid to achieve, and the cure rate is high; meanwhile, the preparation method of the composition is simple, safe, reliable, free of pollution and residues and easy to popularize.
Owner:DALIAN NATIONALITIES UNIVERSITY
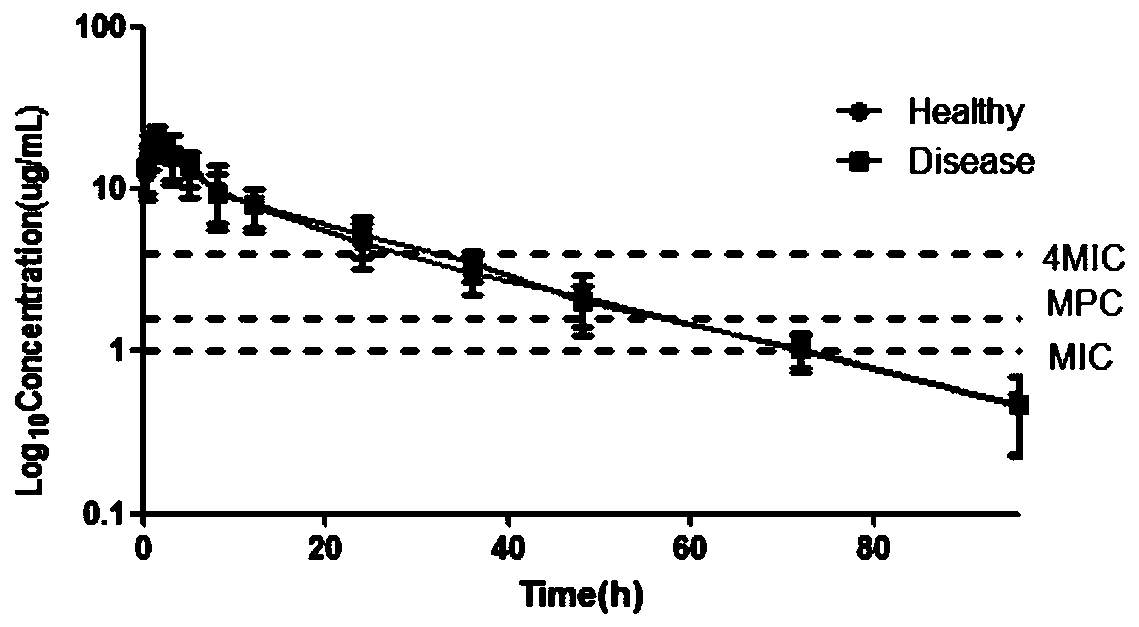
Constructing method of antibiosis medicament ceftiofur pharmacokinetic-pharmacodynamic synchronous model and application thereof
InactiveCN110223734ADelay drug resistanceMedical simulationComputational theoretical chemistryBlood plasmaPK/PD models
The invention discloses a constructing method of an antibiosis medicament ceftiofur pharmacokinetic-pharmacodynamic synchronous model and application thereof. The method comprises the steps of detecting drug susceptibility of the antibiosis medicament ceftiofur to actinobacillus pleuropneumoniae, and obtaining an MIC distribution range; measuring free drug concentrations which are obtained in blood plasma samples which are obtained after drug application to healthy and disease model animals, and obtaining a drug concentration-time curve; fitting the pharmacokinetic parameter of the drug for obtaining a PK parameter; researching an antibiosis function of an antibiosis drug to pathogenic bacteria in in-vitro and semi-in vivo conditions, performing fitting for obtaining a PD parameter; establishing a semi-in vivo PK-PD model; and by means of a dosage calculating formula and Mlxplore software, obtaining a reasonable drug taking plan. The invention provides an optimizing method for an anti-drug-resistance drug taking plan of clinical ceftiofur, thereby alleviating generation and propagation of bacteria drug resistnace, and protecting and sustaining effectiveness of ceftiofur in clinic treatment.
Owner:HUAZHONG AGRI UNIV
Duplex PCR primers for actinobacillus pleuropneumoniae and pasteurella multocida
InactiveCN106987650AEasy to operateStrong characteristicMicrobiological testing/measurementMicroorganism based processesActinobacillus pleuropneumoniaePulmonary infection
The invention provides duplex PCR primers for actinobacillus pleuropneumoniae and pasteurella multocida. The primers are shown as the following: A1:5'-CACGCAGGCGGTTGATTAAG-3', A2:5'-TTGCTACGACTTCACCCCAG-3'; P1:5'-TAAAGCGTGGGGAGCAAACA-3', P2:5'-CAGGCGGTCGATTTATCACG-3'. The invention also establishes a duplex PCR detection method for simultaneous differential diagnosis of actinobacillus pleuropneumoniae and pasteurella multocida, and the method has high accuracy and sensitivity, can achieve precise differential diagnosis of the causes of porcine pulmonary infection, and accordingly implement a right therapeutic schedule to save economic losses.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Preparation method and applications of actinobacillus pleuropneumoniae-derived I-F type CRISPR-associated protein Csy4
InactiveCN106754821AHydrolasesMaterial analysis by electric/magnetic meansEscherichia coliActinobacillus pleuropneumoniae
The invention belongs to the field of genetic engineering, and relates to functions, a preparation method and applications of actinobacillus pleuropneumoniae (App)-derived I-F type CRISPR-associated protein Csy4. The preparation method comprises the following steps: recombining the actinobacillus pleuropneumoniae-derived Csy4 gene onto a pET28b carrier, and constructing a pET28b-Csy4 recombinant plasmid; and converting the pET28b-Csy4 recombinant plasmid into escherichia coli and inducing expression, breaking thalli after expression, centrifuging, and purifying by adopting affinity chromatography technology, so as to obtain Csy4 protein. The inventors find that the Csy4 protein and specific RNA have binding activity by adopting electrophoretic mobility shift assay (EMSA).
Owner:NAT UNIV OF SINGAPORE SUZHOU RES INST
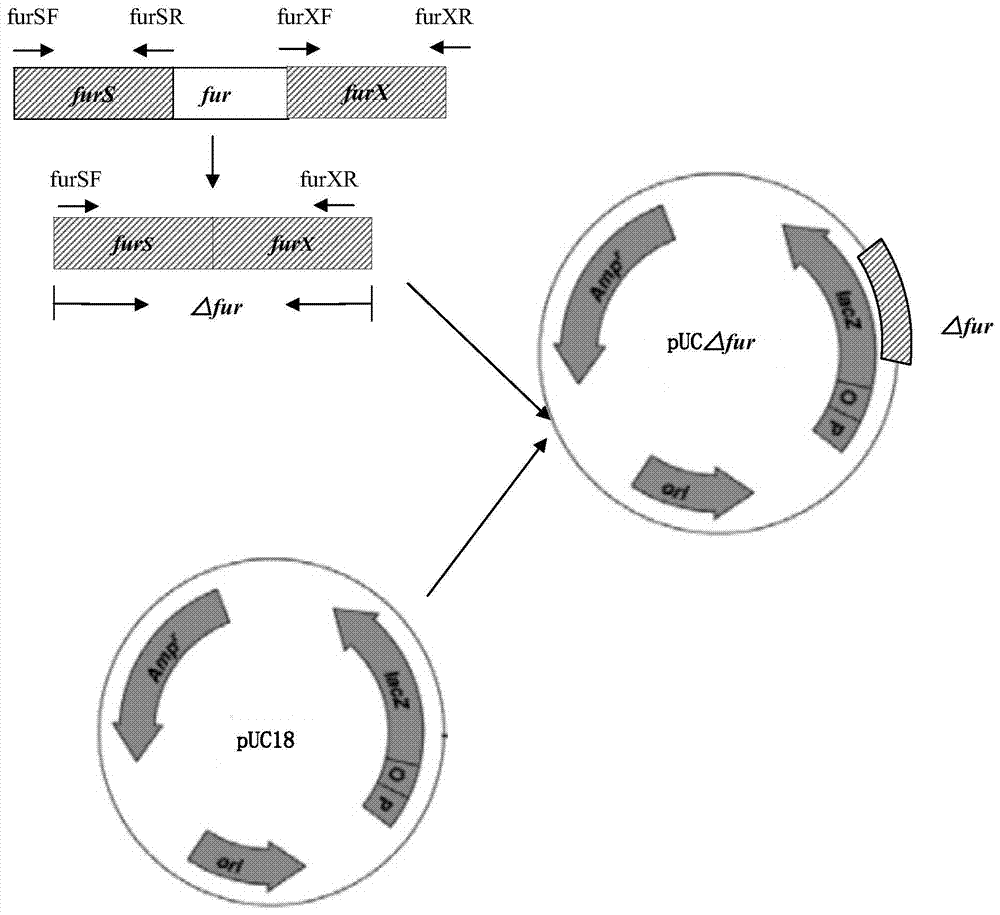
Resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof
InactiveCN103555645BStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsSerum igePleuronectes pinnifasciatus
The invention discloses a resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof and further discloses a resistance marker-free actinobacillus pleuropneumoniae (APP) serum 7 type three gene deletion strain used for preparing the vaccine, belonging to the field of bacterial gene engineering technology. The resistance marker-free APP serum 7 type three gene deletion strain APP-Delta clpP-Delta apxIIC-Delta fur is a strain obtained by inactivating coding genes of protease ClpP, an ApxII toxin activator ApxIIC and an iron absorption regulatory protein Fur of APP through oriented homologous recombination and destroys expression of ClpP, ApxIIC and the Fur protein. The three gene deletion strain obtained in the invention has smaller virulence, decreased by more than 100 times, compared with that of a parent strain, and is safe to animals; A pig immunized by using the strain is well protected from attacks by different serum type virulent strains of APP, and all the protection rates are greater than 80%; and the three gene deletion strain can be used as the attenuated live vaccine for immunoprophylaxis of PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Composition and preparation method for treating swine mycoplasma and porcine pleuropneumonia
ActiveCN105147802BGood treatment effectEasy to prepareAntibacterial agentsAnthropod material medical ingredientsVeterinary DrugsTherapeutic effect
The invention relates to the field of veterinary drugs, in particulrl to a composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and a preparation method thereof. The composition is prepared from, by weight, 15-20 parts of rhizoma atractylodis, 15-20 parts of radix saposhnikoviae, 10-13 parts of liquorice, 10-15 parts of apricot kernels, 30-35 parts of radix astragali, 15-20 parts of radix asteris, 10-15 parts of flos farfarae, 10-15 parts of herba ephedrae, 10-15 parts of periostracum cicada, 10-15 parts of fructus forsythiae and 20-30 parts of lonicera japonica. The composition has the effects of clearing heat, discharging fire, diminishing inflammation, resisting bacteria and the like, all the traditional Chinese medicines are proportioned reasonably to complement and coordinate mutually, the good treatment effect on the swine mycoplasmal pneumonia and the porcine contagious pleuropneumonia is achieved, the treatment course is short, the effects are rapid to achieve, and the cure rate is high; meanwhile, the preparation method of the composition is simple, safe, reliable, free of pollution and residues and easy to popularize.
Owner:DALIAN NATIONALITIES UNIVERSITY
Porcine contagious pleuropneumonia trivalent inactivated vaccine and its preparation method
ActiveCN103212067AEasy to preparePrevention of contagious pleuropneumoniaAntibacterial agentsBacterial antigen ingredientsSerum igeAntigen
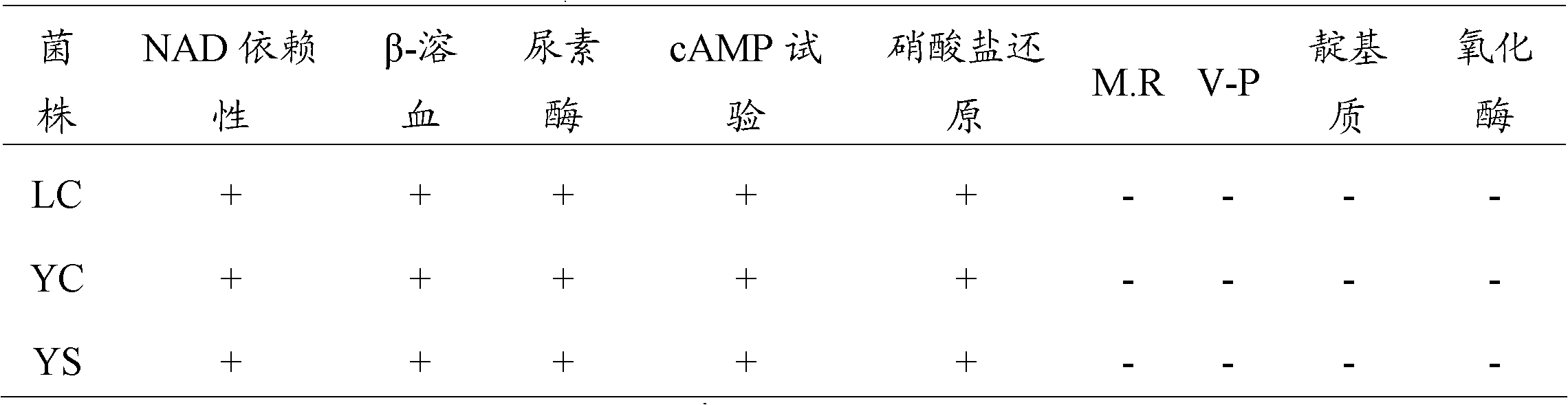
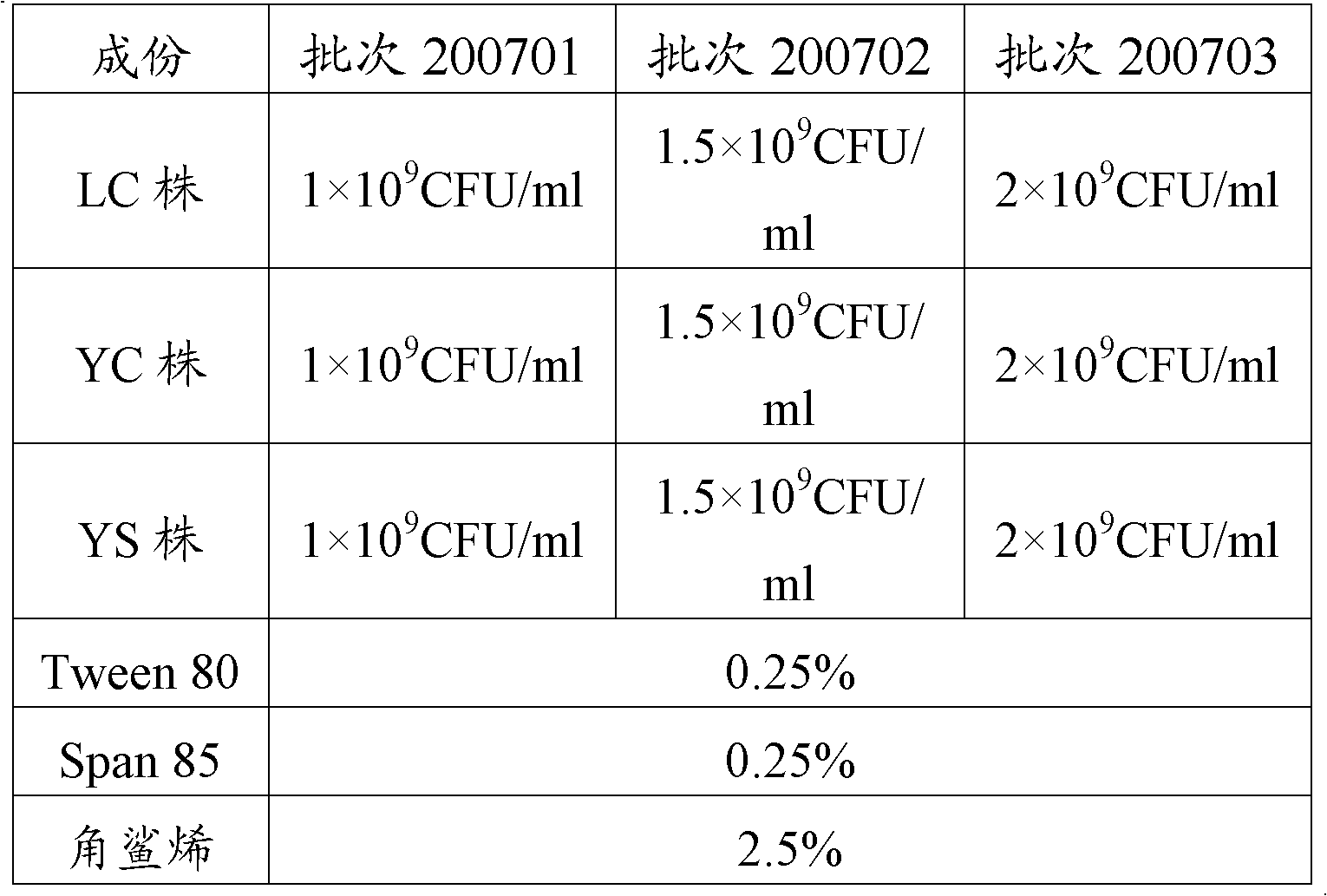
Three porcine contagious Actinobacillus pleuropneumoniae comprising a serotype 1 LC strain, a serotype 5 YC strain and a serotype 7 YS strain are obtained in the invention; and a porcine contagious pleuropneumonia trivalent inactivated vaccine and its preparation method are obtained through the reasonable preparation of inactivated antigens of the above three strains to effectively prevent and control the propagation and the burst of porcine contagious pleuropneumonia in a culturing farm. The invention also provides a porcine contagious pleuropneumonia trivalent inactivated vaccine using an aqueous adjuvant. The porcine contagious pleuropneumonia trivalent inactivated vaccine furthest reduces the stress response during injection, and continuously improves the immune protection effect, so the porcine contagious pleuropneumonia trivalent inactivated vaccine is especially suitable for the large-scale application in Chinese culturing farms.
Owner:PU LIKE BIO ENG
Detection method of actinobacillus pleuropneumoniae in pig serums
InactiveCN103267847AEasy to breedEnhance disease preventionMaterial analysisSerum igeActinobacillus pleuropneumoniae
The invention relates to a detection method of actinobacillus pleuropneumoniae in pig serums. The detection method comprises the steps as follows: randomly selecting serum samples of piglets and finishing pigs in a scale farm, firstly carrying out IHA (indirect hemagglutination test) method detection on the collected serum samples, and secondly carrying out APPELISA detection on the collected serum samples to detect the actinobacillus pleuropneumoniae in the pig serums. According to the detection method, the APP indirect hemagglutination test and the APPELISA test are jointly adopted, so that the detection result of the actinobacillus pleuropneumoniae is accurate, reliable, quick, flexible and stable without error, and the detection method is low in cost, simple in method, suitable for basic level, and brings great convenience for cultivation, disease prevention and disease treatment of pigs.
Owner:TIANJIN TIANZE LIVESTOCK & POULTRY BREEDING SPECIALIZED COOP
Serotyping method of Actinobacillus pleuropneumoniae, primer set and PCR (polymerase chain reaction) systems
ActiveCN110373484ALow costReduce usageMicrobiological testing/measurementMicroorganism based processesActinobacillus pleuropneumoniaeInverse polymerase chain reaction
The invention provides a serotyping method of Actinobacillus pleuropneumoniae, a primer set and PCR (polymerase chain reaction) systems. Specific primers are designed respectively to serotypes 1 to 15of Actinobacillus pleuropneumoniae; the specific primers for the serotypes 9 and 11 have identical sequences, including four groups of 14 pairs of specific primers; each group includes 3 to 4 pairs of specific primers; each PCR system comprises a set of primers, and a multiple PCR system is formed; genome DNA of the serotype of Actinobacillus pleuropneumoniae to be tested is used as a template subjected to PCR detection with the four groups of primers; the results are compared with reference bacterial strips. The primers are designed before being grouped and subjected to multiple PCR; 13 serotypes of Actinobacillus pleuropneumoniae can be identified; high accuracy is achieved at the premise of saving serotype identifying cost, and the detection range is wide.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Vaccine composition and preparation method against swine mycoplasma pneumonia and infectious pleuropneumonia
ActiveCN103623400BReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsSingle injectionSwine Mycoplasma Pneumonia
The invention relates to a vaccine composition against swine mycoplasma pneumonia and infectious pleuropneumonia and a preparation method thereof. The vaccine composition contains Mycoplasma hyopneumoniae antigen and Actinobacillus pleuropneumoniae antigen, has simple immunization procedures, can produce antibodies, and has protection against viruses at the same time, and can effectively prevent and treat Mycoplasma pneumoniae pneumonia and porcine infectious pleuropneumonia. The immune effect of the vaccine composition is at least equal to the effect of separate injection of single vaccine, less side effects, high serum antibody titer, long immunization period, less time-consuming, less labor, and less damage to pigs. The vaccine composition has simple production process, low immunization cost and strong practicability.
Owner:PU LIKE BIO ENG
Actinobacillus pleuropneumoniae serotype 2 bacterial strain and its preparation method
ActiveCN102391975BHigh purityHigh immune protection against virusesBacteriaMicroorganism based processesMonosodium glutamateAntigen
The invention discloses an actinobacillus pleuropneumoniae serotype 2 bacterial strain and its preparation method, which is actinobacillus pleuropneumoniae serotype 2XT, wherein the CCTCC NO. is M2011410. The preparation method comprises the following steps: 1) medium preparation, A) weighting yeast powder, glucose, monosodium glutamate, MgSO4, FeSO4.7H2O, dissolving into pure water, regulating pH value and disinfecting; B) weighting NaH2PO4 and K2HPO4 to prepare mother liquor and disinfecting; C) weighting NAD to prepare mother liquor, filtering by a filter membrane; D) mixing the solutions obtained in the step A, the step B and the step C according to amount to obtain the medium; 2) fermentation and culture, a) activating the freeze-drying seeds by NAD-contained TSA plates until single colony is grown out; b) selecting single colony and culturing by shaking a bottle; c) transferring cultured seed liquid to the fermentation medium; d) low stirring at initial fermentation period, and low ventilating; e) raising rotating speed at logarithmic phase and ventilating, and on-line controlling pH value; f) improving dissolved oxygen, and placing into a tank and collecting bacterium. The actinobacillus pleuropneumoniae serotype 2 bacterial strain suitable for preparing inactivated vaccine has the advantages of strong toxicity, good antigen effect, low price, fast mycelium growth, highdensity and easy control.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
A preparation method of Actinobacillus pleuropneumoniae outer membrane vesicle and vaccine thereof
ActiveCN105420161BExperimental evaluation of immunostimulatory effectsAssessing immunostimulatory effectsAntibacterial agentsBacterial antigen ingredientsIMMUNE STIMULANTSBacteroides
The invention relates to a preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and a vaccine of the APP OMVs, and belongs to the technical field of biology. The preparation method comprises the steps of culturing APP shope strains in vitro by using a culture medium which is in iron ion limit, obtaining acellular cultural supernatant after carrying out centrifugation and 0.22-[mu]m filtration treatment, and preparing the OMVs released by germs through ultracentrifugation, wherein the observation through a transmission electron microscope shows that the diameter of most OMVs is 50 to 100 nm, the OMVs are used as subunit vaccines to carry out secondary intranasal immunization on a mouse, the weight of the OMV immune mouse is increased for a long time, and the visual forms of lungs have no obvious difference from a PBS (Phosphate Buffer Saline) immune group. Meanwhile, an experiment shows that the OMVs are efficient immune stimulants, not only can high-level IgG (Immunoglobulin G) be stimulated to be generated by mouse sera, but also high-level IgA (Immunoglobulin A) can be generated in mouse lungs, mucosal immunity of the mouse lungs is effectively stimulated, and a better application prospect of using the OMVs as the subunit vaccines is expressed.
Owner:JIANGSU ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com