Actinobacillus pleuropneumoniae serotype 2 bacterial strain and its preparation method
A technology of porcine pleuropneumonia and actinobacillus, which is applied in the direction of biochemical equipment and methods, methods based on microorganisms, bacteria, etc., can solve the problems of destruction, poor effect of line protection of porcine pleuropneumonia, low efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: strain isolation and identification
[0045] Pig lungs, throat tonsils and other tissues were aseptically taken from dying disease materials sent for inspection in pig farms, and inoculated on TSA agar medium, 10% CO 2 After culturing at 37°C for 24-36 hours, select a typical single colony for purification and culture. The purified single colony was selected and inoculated in TSB liquid culture medium, and cultivated overnight at 37°C on a shaker (220r / min).
[0046] Culture characteristics: the bacteria in 10% CO 2 , It grows well under the condition of 37 ℃, and grows vigorously on the liquid medium. On TSA (containing NAD) solid medium, in 10% CO 2 , At 37°C, after culturing for 24 hours, transparent and round colonies with a diameter of 1-2 mm are formed.
[0047] Morphological characteristics of the pathogen: Microscopic examination of the lungs and pure cultures of naturally infected pigs and experimentally infected mice showed Gram-negative, str...
Embodiment 2
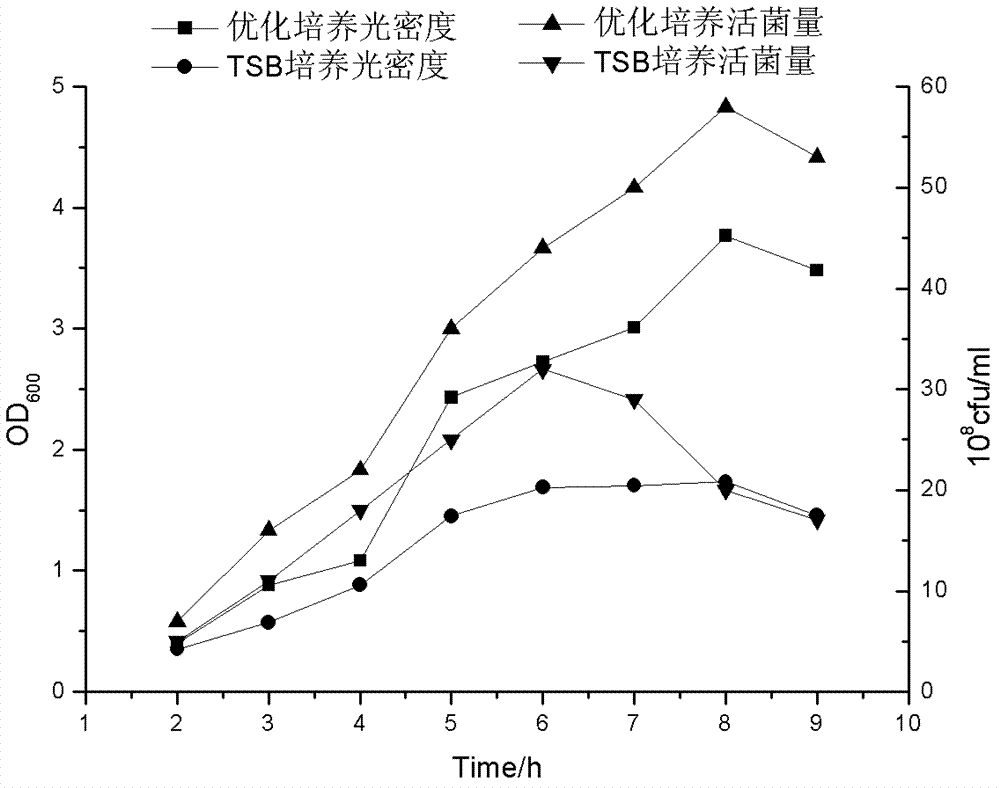
[0055] Embodiment 2: culture medium optimization
[0056] Firstly, the nutrient components of Actinobacillus pleuropneumoniae serotype 2 XT high-density medium were preliminarily screened through a two-level orthogonal test (Table 1, Table 2), and the medium was established in combination with the cost of raw materials and the simplicity of operation. The component factors include: yeast powder, glucose, sodium glutamate, K 2 HPO 4 , NaH 2 PO 4 , MgSO 4 , FeSO 4 ·7H 2 O, coenzyme I, and the four component factors that have the greatest impact on the amount of live bacteria are obtained, in order: yeast powder>K 2 HPO 4 > Sodium glutamate > Glucose, then through the climbing experiment to find the most suitable concentration range of the four most influential components, and then through the central combination experiment and response surface analysis to determine the final concentration. The concentration range of the optimized medium formula obtained from the experime...
Embodiment 3
[0063] Embodiment 3: culture medium preparation
[0064] Medium components and ratio / L
[0065] Glucose 3g; Yeast powder 30g;
[0066] Sodium glutamate 3g; K 2 HPO 4 4.5g;
[0067] NaH 2 PO 4 1g; MgSO 4 0.8g
[0068] FeSO 4 ·7H 2 O 0.1g NAD 0.02g
[0069] Prepared by the following method:
[0070] A. Weigh yeast powder, glucose, sodium glutamate, K 2 HPO 4 , NaH 2 PO 4 , MgSO 4 , FeSO 4 ·7H 2 O. Coenzyme I, set the volume to 900ml, adjust the pH to 7.4, and sterilize under high-pressure steam at 115°C for 25 minutes;
[0071] B. Weigh K by volume 2 HPO 4 , NaH 2 PO 4 , dilute to 100ml, and sterilize under high-pressure steam at 121°C for 15 minutes;
[0072] C. Weigh 2gNAD, set the volume to 100ml, filter through a 0.2μm pore size filter, and take 1ml for later use;
[0073] D. Mix the above three solutions evenly to obtain the XT high-density fermentation medium of Actinobacillus pleuropneumoniae serotype 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com