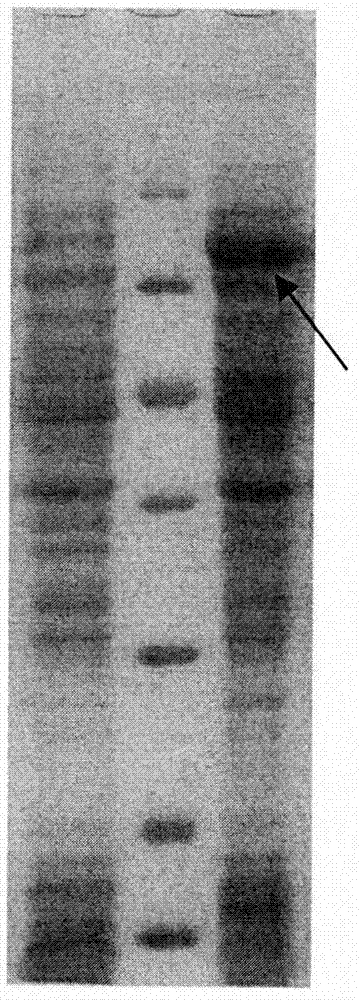
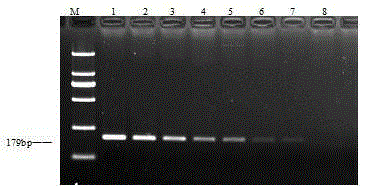
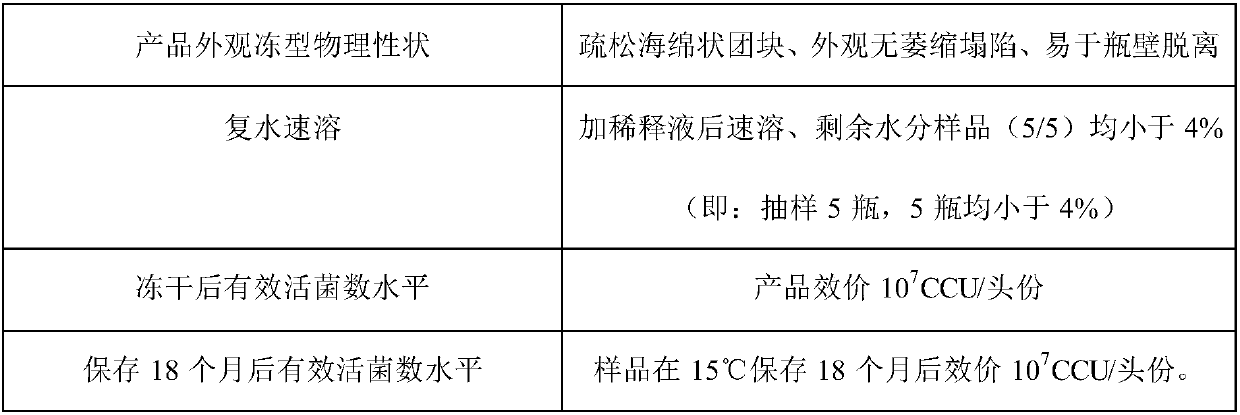
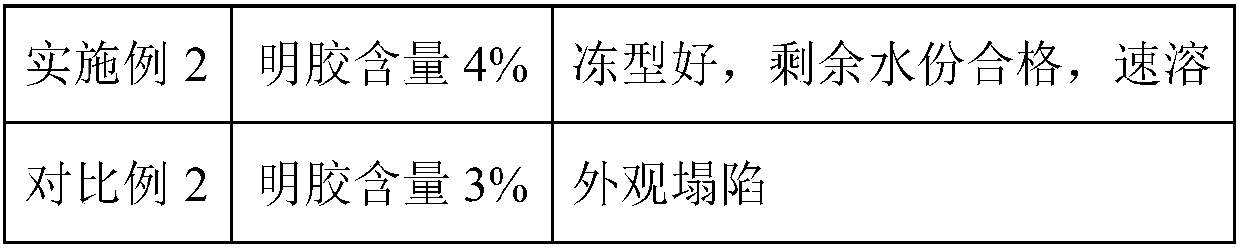
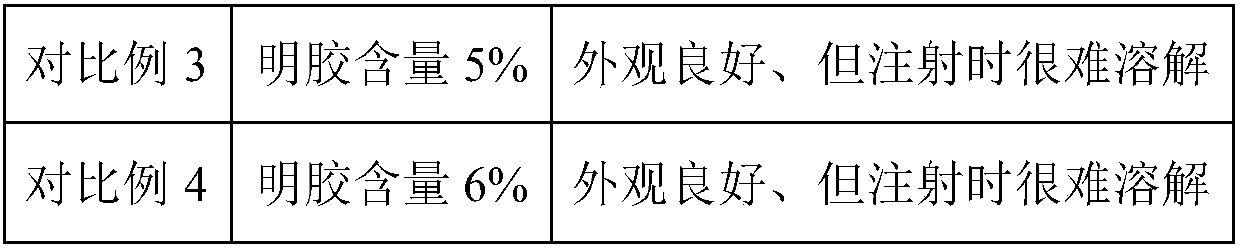
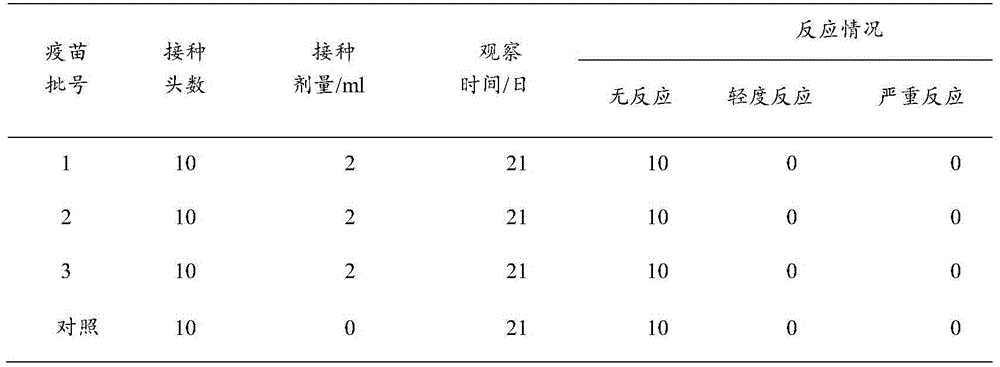
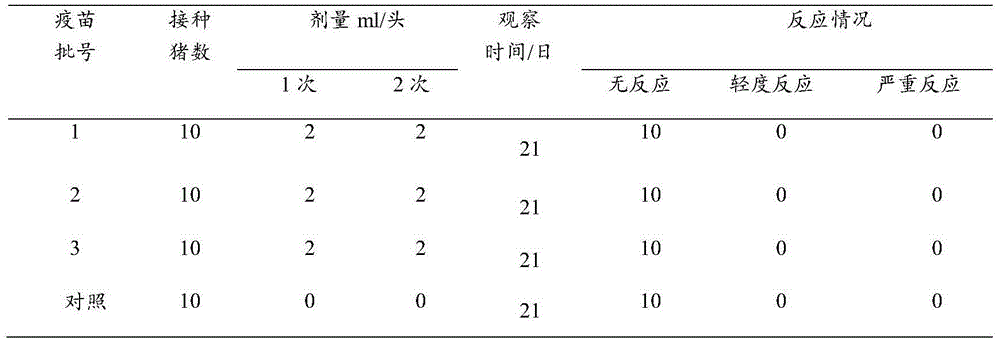
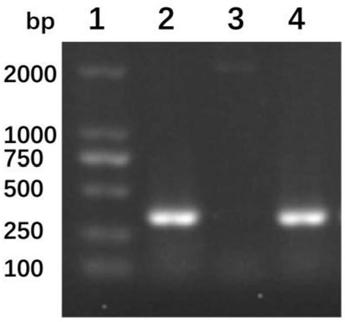
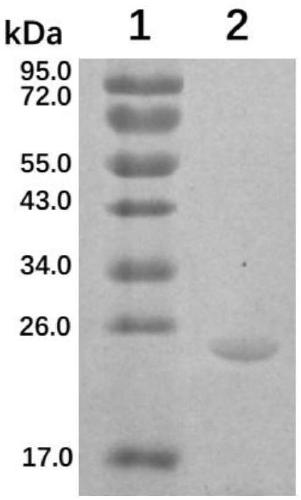
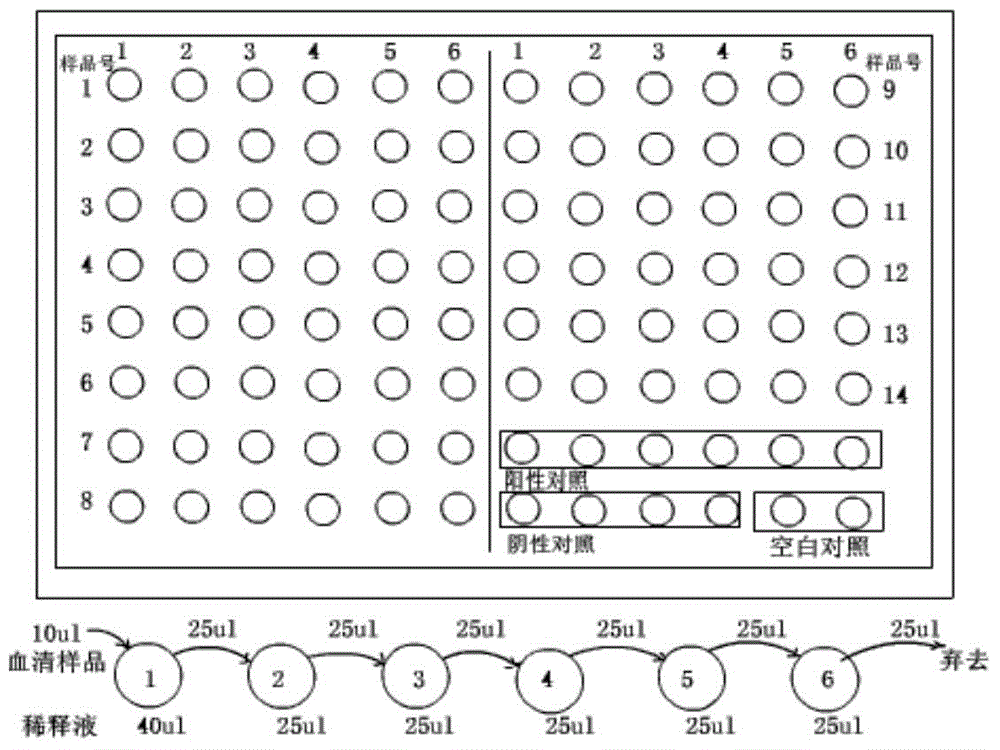
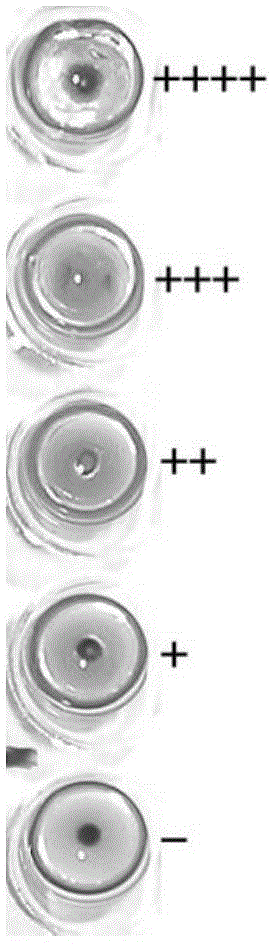
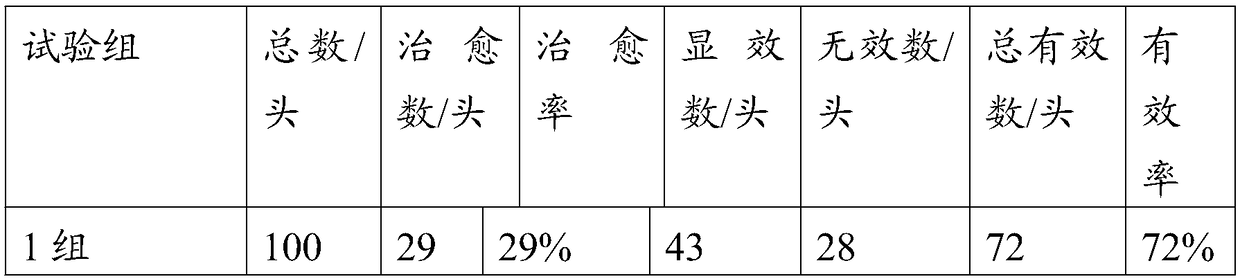
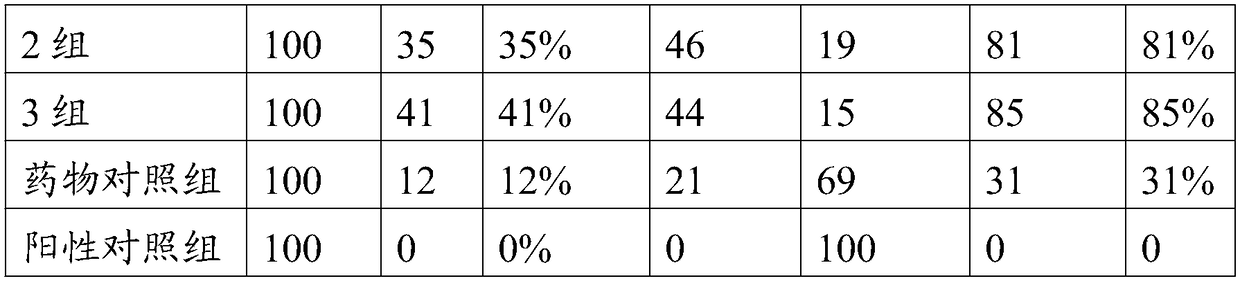
Patents
Literature
55 results about "Swine Mycoplasmal Pneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151AStrengthen cellsEnhance humoral immune stimulationAntibacterial agentsBacterial antigen ingredientsCholesterolVaccine antigen
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Vaccine adjuvant of swine mycoplasmal pneumonia live vaccine, and preparation method and application thereof
ActiveCN101954079AEnhance immune responseImmune activationAntibacterial agentsBacterial antigen ingredientsPhosphateLevamisole
Owner:JIANGSU ACAD OF AGRI SCI
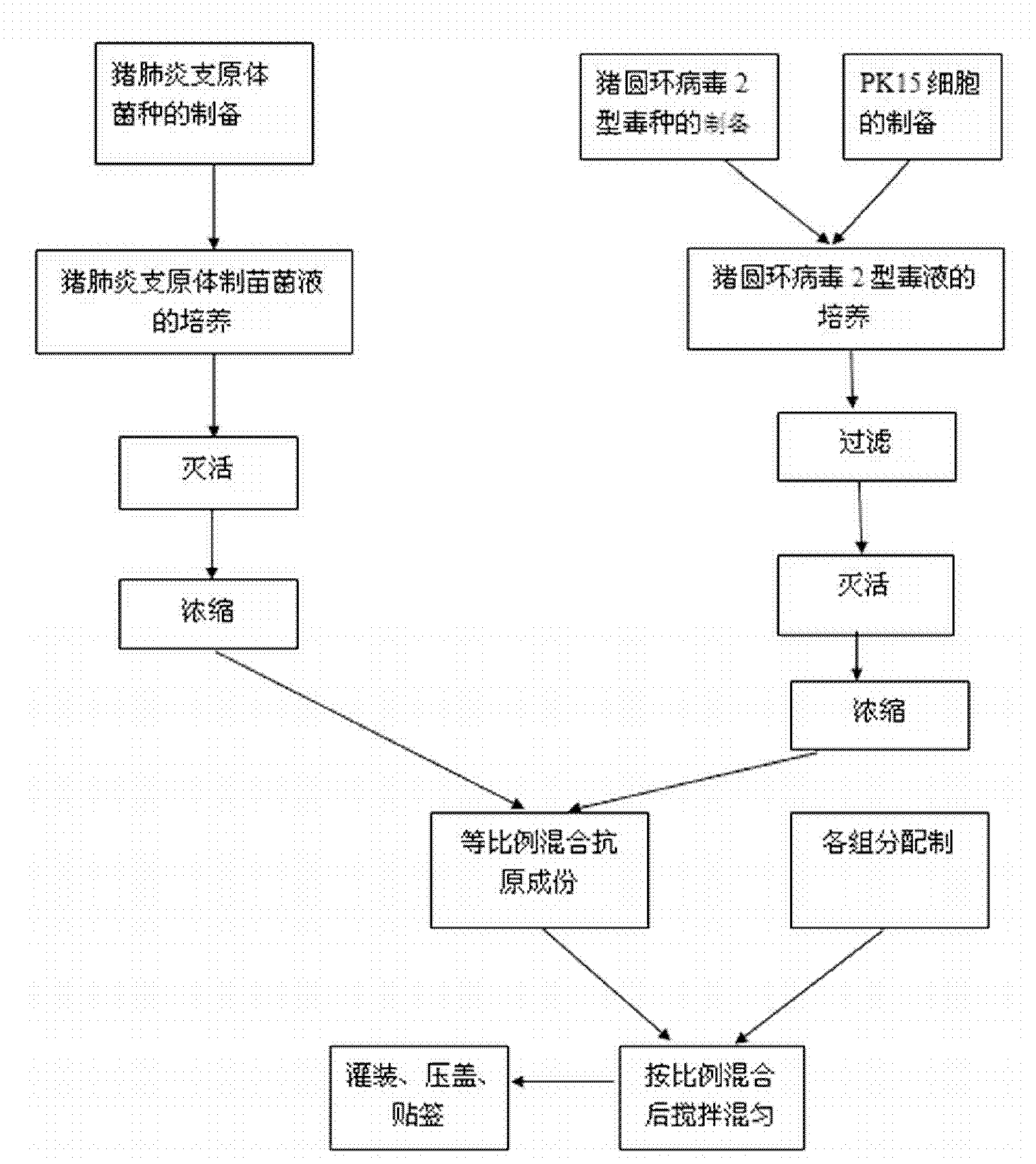
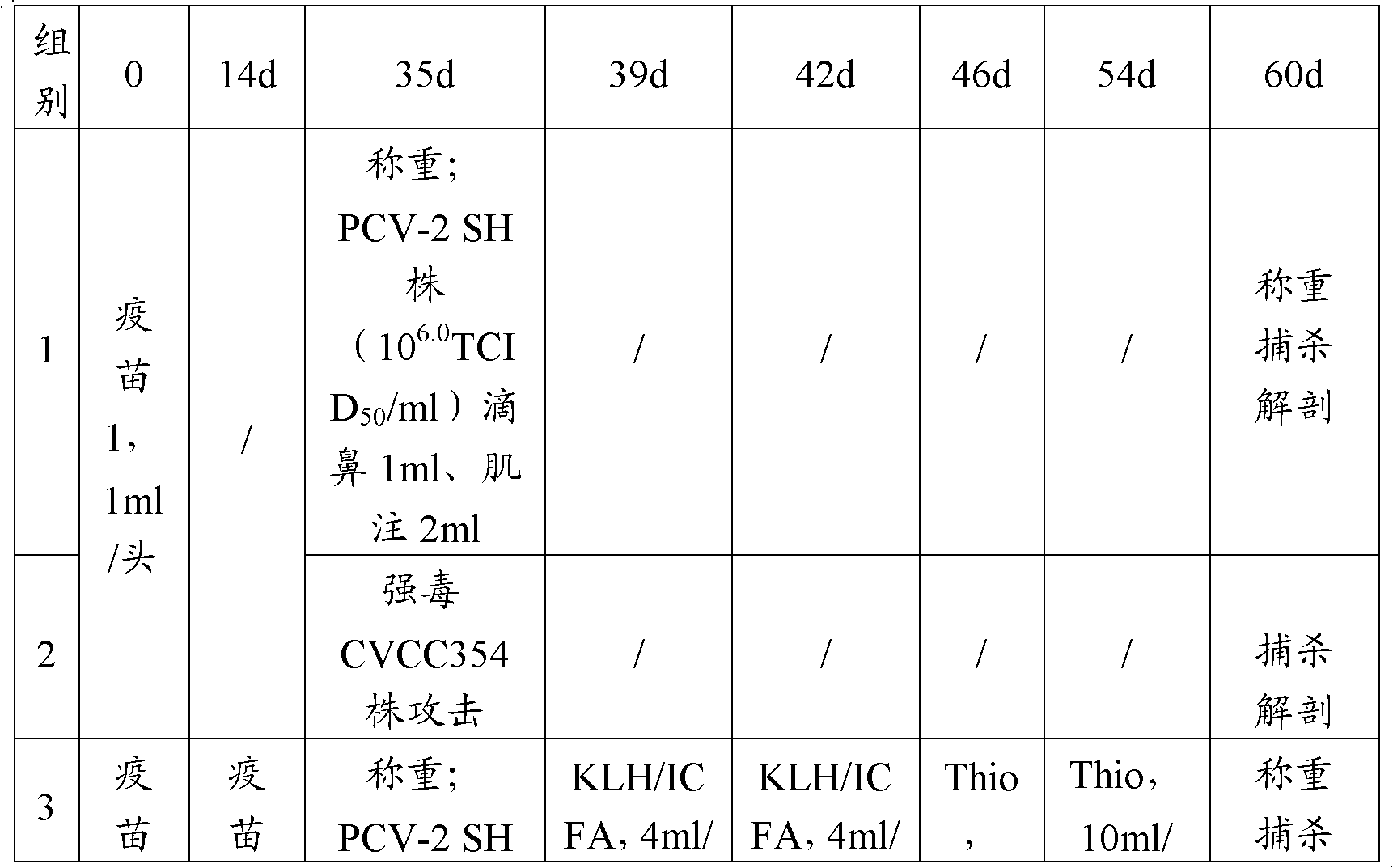
Bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma hyopneumoniae and preparation method of bigeminy inactivated vaccine
ActiveCN103127497ALong-lasting immunityImprove efficiencyAntibacterial agentsViral antigen ingredientsMycoplasma hyopneumoniaeKilled Vaccine
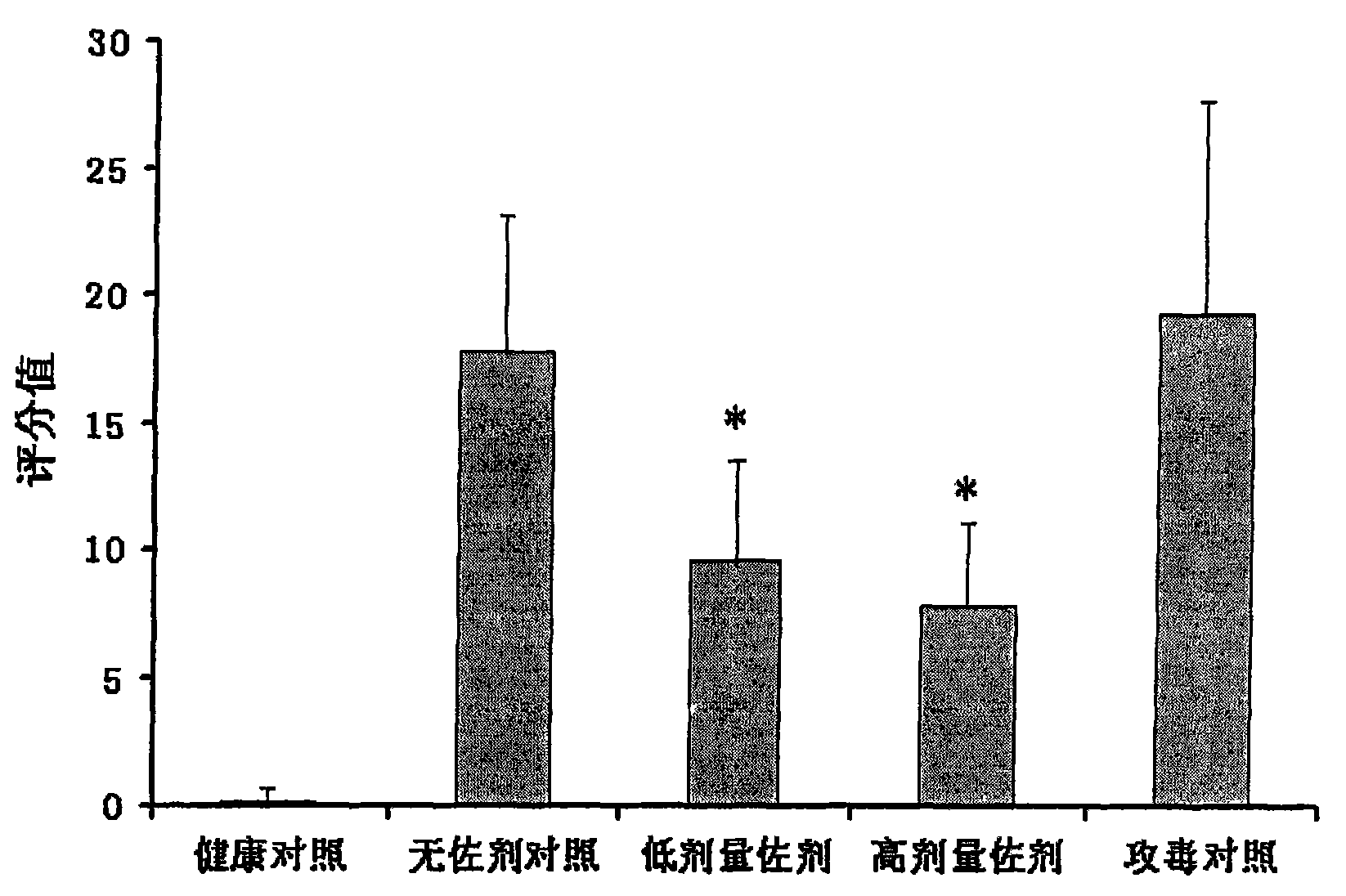
The invention provides a bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma pneumoniae and a preparation method of the bigeminy inactivated vaccine. The bigeminy inactivated vaccine comprises inactivated porcine circovirus type 2, inactivated swine mycoplasma pneumoniae, a vaccine adjuvant, and an excipient, wherein the content of the porcine circovirus type 2 is at least105.5 tissue culture inoculated dose (TCID) 50 / head, and the content of the swine mycoplasma pneumoniae is at least 2*109 MHDCE / head. The bigeminy inactivated vaccine has the advantages that the immune effect is equal to or better than the effect of sum of commodity single vaccines in the market, two antigens do not interfere each other, immune persistent period is long, potency is lasting, and due to the fact that one time immunization only needs, cost is lowered, and stress reaction of animals is also reduced. The bigeminy inactivated vaccine can be used for preventing porcine circovirus disease and at the same time preventing the swine mycoplasma pneumoniae.
Owner:PU LIKE BIO ENG +1
Swine mycoplasmal pneumonia atomized live vaccine and preparation and inspection method thereof
ActiveCN102764431AImprove convenienceLabor savingAntibacterial agentsBacterial antigen ingredientsFreeze-dryingGlycerol
The invention discloses a swine mycoplasmal pneumonia atomized live vaccine which comprises vaccine strains and vaccine diluent. The vaccine strains are freeze-dried strains with mycoplasma hyopneumoniae attenuated strains (168 strains) or freshly prepared bacterial liquid. The vaccine diluent is deionized water solution with the pH (potential of hydrogen) value of 6.8-7.5 and containing 5-10% of glycerol in final concentration, or is deionized water solution containing 0.01-0.1% of propylene glycol block polyether (F68), or is deionized water solution containing 0.1% of polyvinyl pyrrolidone (PVP), or is an optional combination of protective agents. Besides, the invention further discloses a preparation and inspection method of the vaccine. The swine mycoplasmal pneumonia live vaccine is atomized, convenience in vaccination can be improved, labor power and labor time are greatly saved, the vaccine can be more effectively popularized for immunity, and the vaccine is applicable to the technical field of medicinal preparations.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
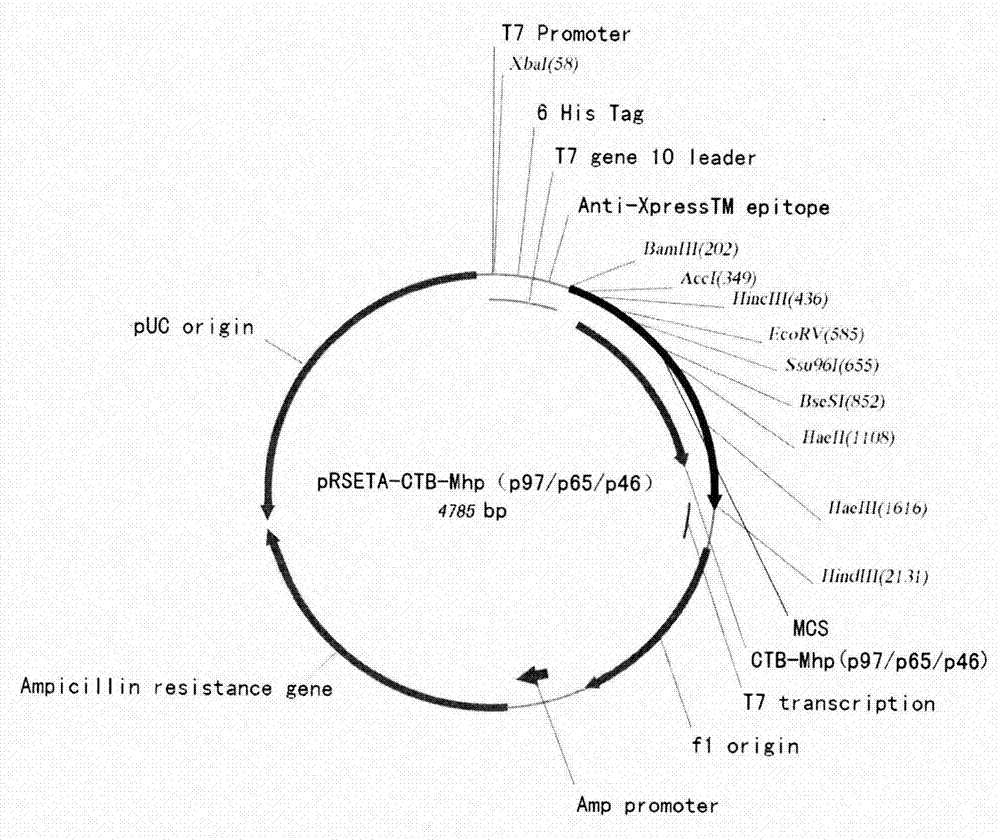
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
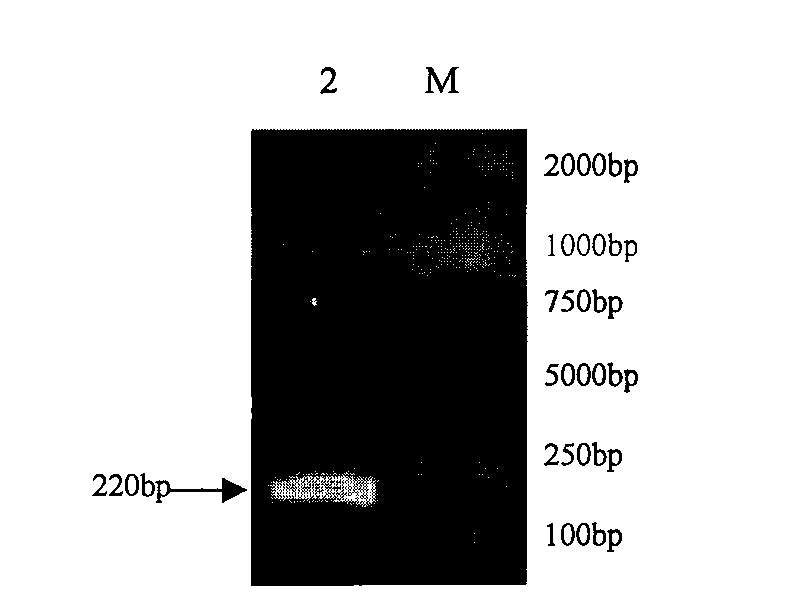
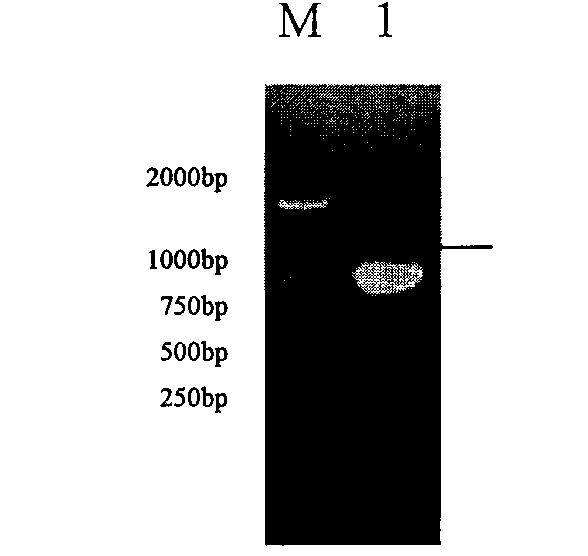
Mycoplasma hyopneumoniae loop-mediated isothermal amplification kit and application thereof
PendingCN105349672ANo pollutionAvoid pollutionMicrobiological testing/measurementMicroorganism based processesSwine Mycoplasmal PneumoniaLesion
The invention discloses a mycoplasma hyopneumoniae loop-mediated isothermal amplification kit and the application thereof. The kit comprises LAMP primers, 2x reaction buffer, Bst DNA polymerase, a fluorescence visual detection reagent, ultrapure water and a mycoplasma hyopneumoniae DNA template, wherein the LAMP primers include outer primers F3 and B3, inner primers FIP and BIP, and loop primers LF and LB. The kit can be applied to detection of lesion tissue of suspected mycoplasma pneumoniae of swine and a mycoplasma hyopneumoniae culture. Specificity detection and sensitivity detection prove that by the adoption of the LAMP detection method, reaction can be monitored in real time, the mycoplasma hyopneumoniae copy number can be detected, a detection result can be obtained quickly and accurately, and convenience is brought to easy, quick and reliable detection of mycoplasma hyopneumoniae.
Owner:GUANGXI VETERINARY RES INST
Method for quick freeze drying of swine mycoplasmal pneumonia live vaccines for 50 pigs
ActiveCN107693497AShort freeze-drying run timesReduce energy consumptionAntibacterial agentsPowder deliveryAntigenQuick Freeze
The invention relates to the technical field of vaccine freeze drying and particularly relates to a method for quick freeze drying of swine mycoplasmal pneumonia live vaccines. The method comprises the following steps: (1) reviving and subculturing the freeze-dried strains of swine mycoplasmal pneumonia live vaccines, and selecting a product freeze-drying antigen material; (2) preparing a gelatinsucrose freeze-drying protection agent; (3) mixing the antigen and the protection agent fully and uniformly; (4) quantitatively packaging the fully mixed product, and putting on the plate layers of a freeze drying box; (5) running a freeze drying program: (i) freezing: on under the condition of refrigerating the plate layers at 5 DEG C, cooling to -40 DEG C within 1 hour, and maintaining for 4 hours; (ii) vacuumizing: vacuumizing the freeze drying box so that the pressure is below 0.1mbar; (iii) sublimation drying: on under the condition of 0.07mbar of vacuum, heating to 10 DEG C within 1 hour,and maintaining for 15 hours; then, further maintaining for 1 hour at 15 DEG C; (iv) secondary drying: heating to 32 DEG C in 20 minutes, and maintaining for 5 hours; further heating to 36 DEG C within 20 minutes, and maintaining for 2 hours; (v) starting a plugging function to plug the freeze-dried vaccines, and discharging out of the box.
Owner:兆丰华生物科技(南京)有限公司 +1
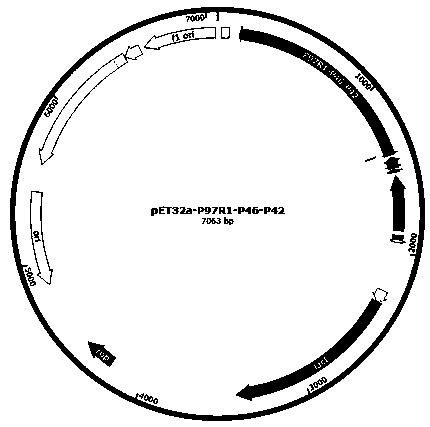
Recombinant protein of Mycoplasma pneumonia and porcine circovirus type 2 and bivalent vaccine prepared therewith
ActiveCN109207502AAntibacterial agentsAntibody mimetics/scaffoldsPorcine circovirusGenetically engineered
The invention relates to the technical field of vaccines, in particular to a recombinant protein of mycoplasma suis pneumonia and porcine circovirus type 2, a bivalent vaccine prepared therewith and application thereof. A Mycoplasma pneumonia and porcine circovirus type 2 bivalent genetically engineered vaccine is characterized in that the vaccine includes Mhp P97R1-P46-P42 recombinant protein andPCV2 Cap recombinant protein. The encoding gene sequence of the Mhp P97R1-P46-P42 recombinant protein is shown in SEQ ID NO. 1; The coding gene sequence of the PCV2 Cap recombinant protein is shown in SEQ ID NO. 2. The bivalent genetically engineered vaccine can effectively prevent and control Mycoplasma pneumonia and porcine circovirus type 2, and has good application prospect.
Owner:浙江洪晟生物科技股份有限公司
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258BImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
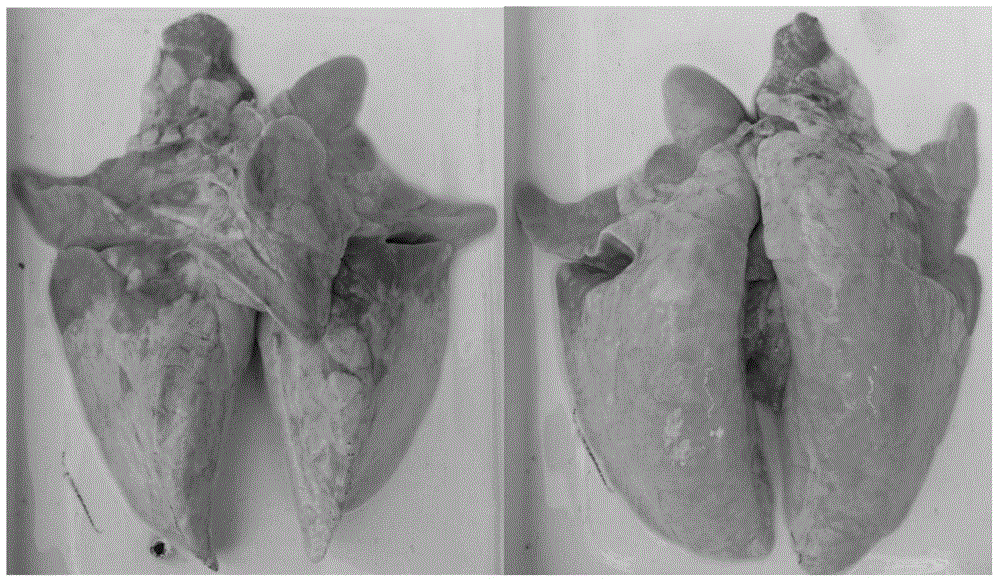
Attenuated live vaccine against mycoplasmal pneumonia of swine (MPS) and use thereof
ActiveUS20160346372A1Prevention and control of diseaseEffectively activate the immune systemAntibacterial agentsBacterial antigen ingredientsAdjuvantLung tissue
Disclosed are an attenuated live vaccine against mycoplasmal pneumonia of swine (MPS) and use thereof. In the present invention, pathological lung tissues of swine having typical Mycoplasma hyopneumoniae (Mhp) infection and no obvious other pathogenic infections are screened, and subcultured 100 generations in lungs of newborn rabbits; then, Mhp strains are isolated and serially subcultured in a medium; and the Mhp strain AN306 is obtained by screening a plurality of strains, which is deposited with an accession number: CCTCC NO. M2014176. Also disclosed is a live vaccine formulation against MPS prepared on the basis of the attenuated strain and comprising live attenuated strain, a pharmaceutically acceptable carrier or excipient, and optionally an adjuvant and immunogens of other pathogens.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Swine mycoplasmal pneumonia prevention and treatment traditional Chinese medicine extract preparation and preparation method thereof
InactiveCN103463279AInhibition of killingEasy to useAmphibian material medical ingredientsRespiratory disorderBiotechnologyHouttuynia
The present invention provides a swine mycoplasmal pneumonia prevention and treatment traditional Chinese medicine extract preparation and a preparation method thereof, wherein 25-35 parts by weight of loquat leaf, 15-25 parts by weight of heartleaf houttuynia herb, 18-22 parts by weight of ephedra, 12-18 parts by weight of balloonflower root, 12-18 parts by weight of honeysuckle, and 0.5-1.5 parts by weight of dried toads venom are subjected to sorting, ultrafine crushing, soaking extraction, secondary soaking extraction, drug liquid mixing, concentration, filtration and sub-packaging to obtain the preparation. According to the present invention, the preparation is diluted by using clear water according to a ratio of 1:100, pigs drink the obtained diluted preparation 2 times a day, and the whole process is 3-5 days, such that swine mycoplasmal pneumonia can be effectively controlled; and the preparation has characteristics of easy use, low cost, no residue, and high biological safety.
Owner:ZHENJIANG TIANHE BIOLOGICAL TECH
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151BStrengthen cellsImprove the effectiveness of anti-virus protectionAntibacterial agentsBacterial antigen ingredientsCholesterolLevamisole
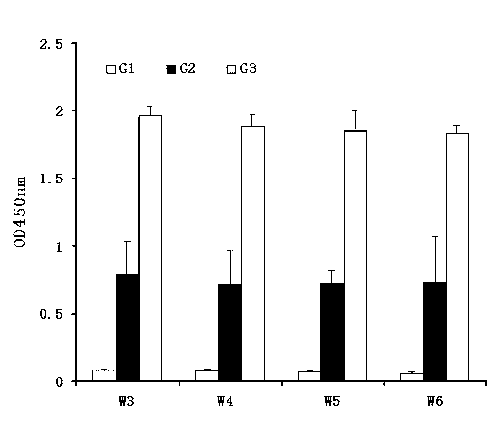
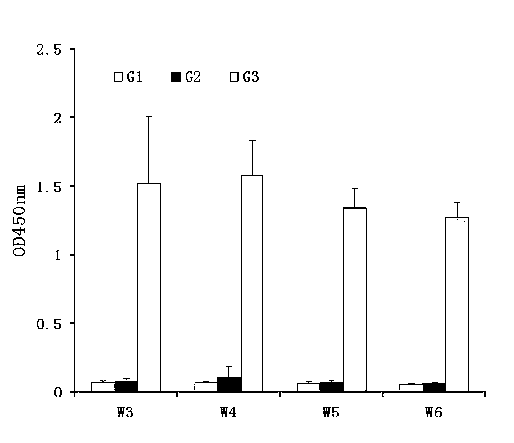
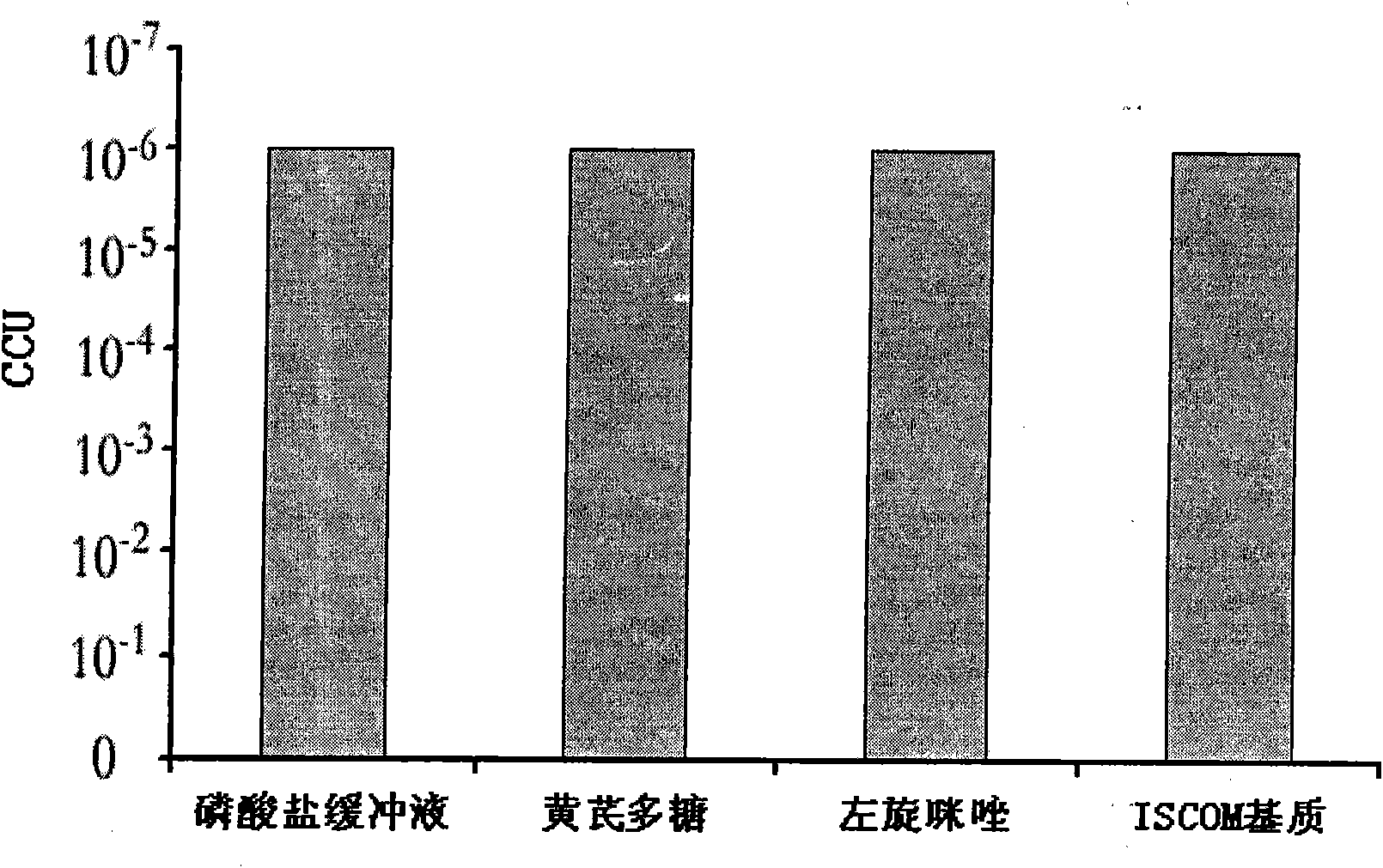
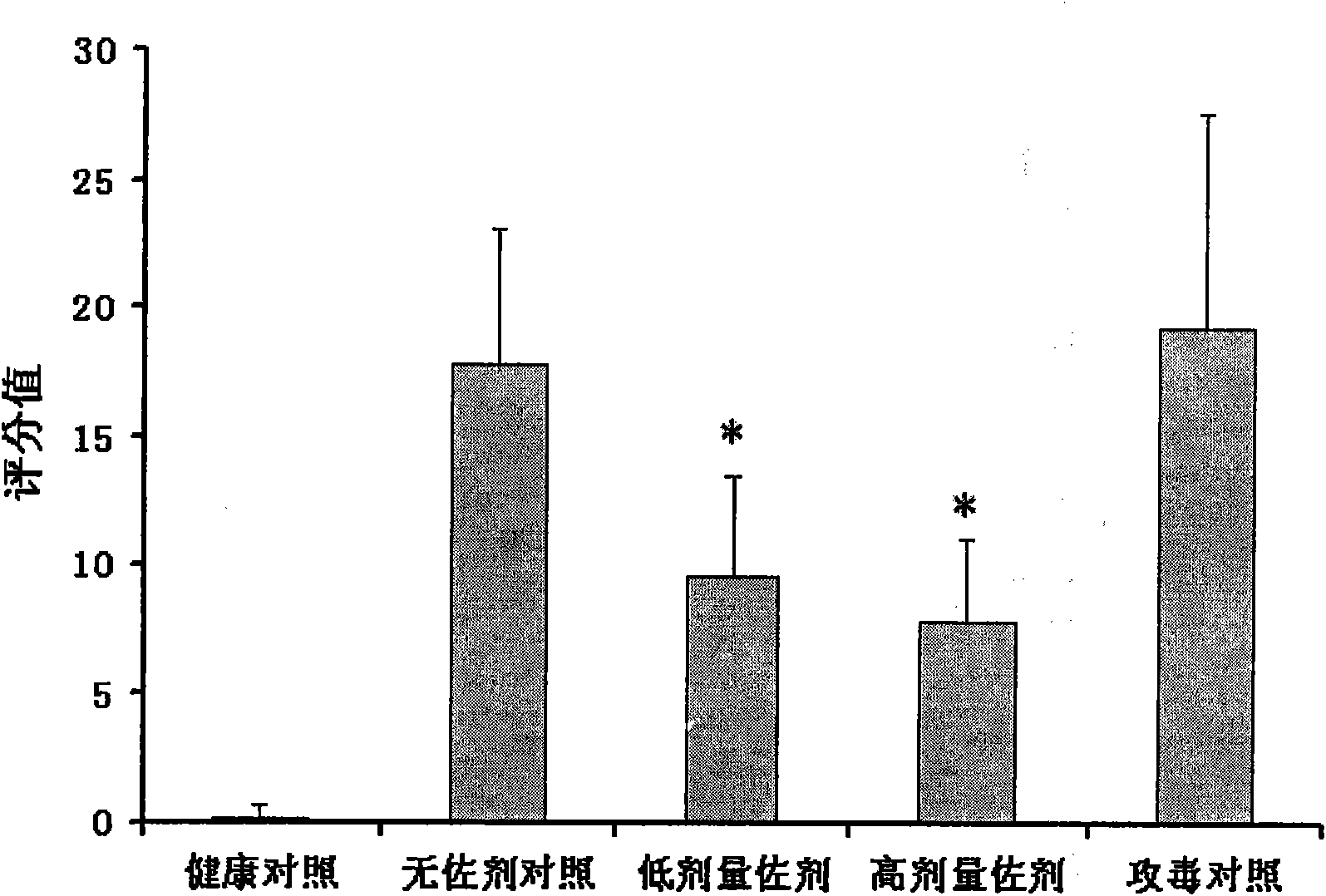
The special diluent for swine mycoplasma pneumonia vaccine of the present invention mainly contains recombinant P97R1 protein immunostimulatory complex (ISCOM-P97R1), and may also contain other aqueous adjuvant components, including levamisole, or / and astragalus polysaccharide, or / and carboxy bohm. Each ml solution of the diluent contains 0.1-1 mg of QuilA, 0.02-0.2 mg of cholesterol, 0.02-0.2 mg of phosphatidylcholine, 0.4-4 mg of P97R1 antigen, and may further contain 2-20 mg of levamisole , or / and astragalus polysaccharide 20-100 mg, or / and carbomer 3-10 mg, and the rest is phosphate buffer. This diluent is specially used for dissolving or diluting Mycoplasma pneumoniae vaccine, including live vaccine and inactivated vaccine. It has the dual effects of supplementing the protective spectrum of vaccine antigens and enhancing the immune stimulating ability of vaccine cells and humoral, which can significantly improve the protective efficacy of vaccines against viruses. At the same time, due to the use of the water solvent system, it also has the advantages of simple operation, good needle penetration, and no obvious toxic and side effects.
Owner:JIANGSU ACAD OF AGRI SCI
A kind of aerosolized live vaccine of swine mycoplasma pneumonia and its preparation and testing method
ActiveCN102764431BImprove convenienceLabor savingAntibacterial agentsBacterial antigen ingredientsFreeze-dryingGlycerol
The invention discloses a swine mycoplasmal pneumonia atomized live vaccine which comprises vaccine strains and vaccine diluent. The vaccine strains are freeze-dried strains with mycoplasma hyopneumoniae attenuated strains (168 strains) or freshly prepared bacterial liquid. The vaccine diluent is deionized water solution with the pH (potential of hydrogen) value of 6.8-7.5 and containing 5-10% of glycerol in final concentration, or is deionized water solution containing 0.01-0.1% of propylene glycol block polyether (F68), or is deionized water solution containing 0.1% of polyvinyl pyrrolidone (PVP), or is an optional combination of protective agents. Besides, the invention further discloses a preparation and inspection method of the vaccine. The swine mycoplasmal pneumonia live vaccine is atomized, convenience in vaccination can be improved, labor power and labor time are greatly saved, the vaccine can be more effectively popularized for immunity, and the vaccine is applicable to the technical field of medicinal preparations.
Owner:JIANGSU ACAD OF AGRI SCI
Agent for protecting pig mycoplasma vaccine and promoting absorption as well as production method and use method thereof
InactiveCN101376024AExtended stayImprove drug absorptionAntibacterial agentsBacterial antigen ingredientsSpray bottleKilled Vaccine
A protective absorbent for a vaccine against swine mycoplasma pneumonia is prepared from carbomer as a base material, Tween-80 as an absorption promoting agent, a small amount of blood serum, and normal saline as a diluent. The application method of the protective absorbent comprises the steps of mixing the protective absorbent with an inactivated vaccine against swine mycoplasma pneumonia according to a volume ratio of 1:30, loading into a spray bottle, sufficiently shaking, and spraying into the nasal cavity of swine. The vaccine inoculation method prolongs the retention time of medicinal ingredients in the nasal cavity of swine, improves absorption rate and capacity of medicinal ingredients, and reduces nasal cilia clearance ratio. Compared with conventional intrapleural injection and intramuscular injection, the vaccine inoculation simplifies the application method of the vaccine against swine mycoplasma pneumonia and improves the epidemic prevention efficiency, with no significant difference with respect to protection ratio.
Owner:SHANXI HAISEN BIOLOGIC PRODS
Vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and preparation method
ActiveCN103623400AReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsTGE VACCINEMycoplasma pneumonia
The invention relates to a vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and a preparation method. The vaccine composition comprises pig mycoplasma hyopneumoniae antigens and pig actinobacillus pleuropneumoniae antigens, is simple in immunization procedure, not only is capable of generating antibodies, but also has the functions of toxicity attack protection and effectively controlling pig mycoplasma pneumonia and infectious pleuropneumonia. The vaccine composition has the immunization effect same to an independently injected vaccine, is small in side reaction, long in immunization period, less in consumed time, less in consumed labor, small in encroaching on a pig. The vaccine composition is simple in production technology, low in immunization cost and strong in practicality.
Owner:PU LIKE BIO ENG
Porcine reproductive and respiratory syndrome, swine mycoplasmal pneumonia combined live vaccine and preparation method thereof
InactiveCN107349424ASecurity plusEasy to prepareAntibacterial agentsPowder deliveryFreeze-dryingVaccine antigen
The invention relates to porcine reproductive and respiratory syndrome, swine mycoplasmal pneumonia combined live vaccine and a preparation method thereof, which belong to the technical field of a veterinary biological product. The vaccine contains swine mycoplasmal pneumonia live vaccine antigen with immunization amount and porcine reproductive and respiratory syndrome with immunization amount, a freeze-drying protective additive is added, and a step of freeze drying is carried out. The antigenic component of the combined live vaccine are non-mutual interference or non-mutual influence, and mutually enhance the immunization effect, the primary immunization can reach the immunization protection, and the method has the advantages of good security, simple preparation method, convenient and fast immunization, and reduced immunization cost.
Owner:NANJING DAYAO NETWORK TECH CO LTD
Traditional Chinese medicine composition for preventing and treating swine mycoplasmal pneumonia and preparation method of traditional Chinese medicine composition
InactiveCN103768239ANo residueMoisturizing the lungs and relieving coughAntibacterial agentsRespiratory disorderLithospermumLiquorices
The invention relates to a traditional Chinese medicine composition for preventing and treating swine mycoplasmal pneumonia. The traditional Chinese medicine composition comprises the ingredients in parts by weight: 5-20 parts of gypsum, 10-40 parts of coptis root, 10-35 parts of coptis root, 10-35 parts of radix scutellariae, 5-25 parts of platycodon grandiflorum, 10-40 parts of liquorice, 20-45 parts of radix scrophulariae, 15-45 parts of lithospermum, 10-30 parts of feltwort, 20-50 parts of ginkgo and 5-30 parts of perilla leaf. The composition is scientific and reasonable in formulation, convenient to use and lower in cost, and a preventing and treating rate of the swine mycoplasmal pneumonia is approximately 90%.
Owner:TIANJIN ZHONGAO BIOTECH
Traditional Chinese medicine composition for treating swine mycoplasmal pneumonia and preparation method thereof
InactiveCN104353053APreserve the combination effectWith expectorant benefitAntibacterial agentsPowder deliverySide effectGleditsia triacanthos
The invention relates to a traditional Chinese medicine composition for treating swine mycoplasmal pneumonia. The traditional Chinese medicine composition is prepared from cassia twig, ginger, liquorice, jujube, Chinese honey locust, platycodon grandiflorum and semen lepidii. The traditional Chinese medicine composition can be powder and is prepared according to the following steps: (1) weighing the traditional Chinese medicines according to the amount of a formula; (2) crushing the traditional Chinese medicines to 20-100 meshes; (3) mixing for 10-20 minutes to obtain traditional Chinese medicine powder; (4) separately packaging the traditional Chinese medicine powder at a dosage of 100g to 200g every bag to obtain a finished product. The traditional Chinese medicine composition has the effects of eliminating phlegm, promoting flow of qi and relieving cough and asthma. The traditional Chinese medicine composition provided by the invention is prepared from wide raw material sources, low in cost, free of side effects and medicine residues, safely applicable to treatment on the swine mycoplasmal pneumonia and higher than 95% in effective rate.
Owner:TIANJIN DAYS NONGDA BIOTECH DEV LIMITED
Swine mycoplasma pneumonia inactivated vaccine and preparation method thereof
ActiveCN104399070AGood spiritsNormal feedingAntibacterial agentsBacterial antigen ingredientsAdjuvantAntibody level
The invention discloses a swine mycoplasma pneumonia inactivated vaccine and a preparation method thereof and belongs to the field of swine mycoplasma pneumonia inactivated vaccines. The swine mycoplasma pneumonia inactivated vaccine comprises a water phase and an oil phase according to a volume ratio of 1: 1-3. The water phase comprises Tween-80 and swine pneumoniae mycoplasma liquid according to a ratio of 2-5: 95-98. The oil phase comprises polyoxyl-40-hydrogenated castor oil, Span-80 and white oil according to 1-2: 5-8: 90-94. The invention further discloses a method for preparing the swine mycoplasma pneumonia inactivated vaccine. An emulsification adjuvant of the swine mycoplasma pneumonia inactivated vaccine is added with polyethyleneglycol-40-hydrogenated castor oil with hydrophilic and hydrophobic groups, substantially improves vaccine emulsification effects, substantially reduces a use amount of white oil and vaccine consistency, reduces injection difficulty and animal stress response, realizes a high antibody level in a long period and has substantial immunization effects on piglets.
Owner:哈药集团生物疫苗有限公司
Swine Mycoplasmal pneumonia live vaccine mucosal immune adjuvant, preparation method and application thereof
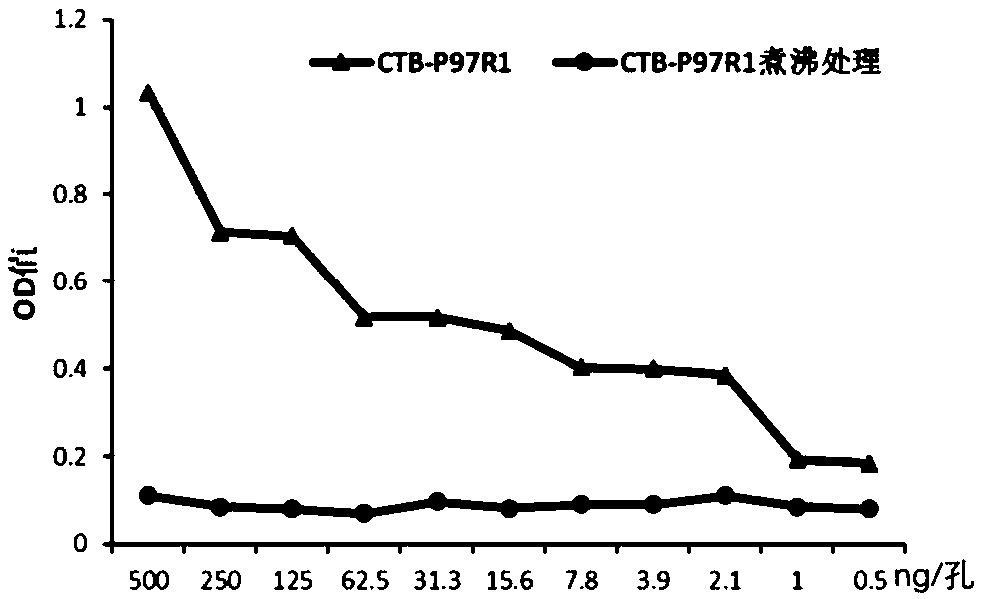
ActiveCN109200284AEasy to prepareMeet the need for immune protectionAntibacterial agentsBacterial antigen ingredientsMucosal adjuvantSolvent
The invention provides a swine Mycoplasmal pneumonia live vaccine mucosal immune adjuvant, a preparation method and application thereof, which relate to the field of veterinary vaccines. The mucosal adjuvant is a solution of a recombinant protein CTB-P97R1 containing fusion expression of cholera toxin B subunit and Mycoplasma hyopneumoniae P97R1. The invention also provides the preparation methodof the mucosal adjuvant, wherein preparing 10 mg / ml recombinant protein CTB-P97R1 mother liquor, then diluting the mother liquor with a solvent. The invention also provides the application of the mucosal immune adjuvant in preparing a live vaccine of Mycoplasmal pneumonia . The mucosal adjuvant of that invention is an aqueous compound adjuvant, The preparation method is simple, the adjuvant property is stable, the mycoplasma hyopneumoniae activity is not damaged, and the mixture is compatible with aerosol protectant, and the mixture is still harmless to mycoplasma hyopneumoniae activity, so itis suitable for aerosol immunization of live vaccine. Using the adjuvant can significantly improve the mucosal immune protection efficacy of vaccine, and prolong the immune duration.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and preparation method
ActiveCN105147802AEffective treatmentHas the effect of detoxifying and relieving asthmaAntibacterial agentsAnthropod material medical ingredientsVeterinary DrugsTherapeutic effect
The invention relates to the field of veterinary drugs, in particulrl to a composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and a preparation method thereof. The composition is prepared from, by weight, 15-20 parts of rhizoma atractylodis, 15-20 parts of radix saposhnikoviae, 10-13 parts of liquorice, 10-15 parts of apricot kernels, 30-35 parts of radix astragali, 15-20 parts of radix asteris, 10-15 parts of flos farfarae, 10-15 parts of herba ephedrae, 10-15 parts of periostracum cicada, 10-15 parts of fructus forsythiae and 20-30 parts of lonicera japonica. The composition has the effects of clearing heat, discharging fire, diminishing inflammation, resisting bacteria and the like, all the traditional Chinese medicines are proportioned reasonably to complement and coordinate mutually, the good treatment effect on the swine mycoplasmal pneumonia and the porcine contagious pleuropneumonia is achieved, the treatment course is short, the effects are rapid to achieve, and the cure rate is high; meanwhile, the preparation method of the composition is simple, safe, reliable, free of pollution and residues and easy to popularize.
Owner:DALIAN NATIONALITIES UNIVERSITY
Vaccine composition containing swine mycoplasmal pneumonia antigen and swine streptococcosis antigen, and preparation method and application thereof
ActiveCN103861095APreserve immune efficiencyLow costAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
Recombinant adenovirus expressing mycoplasma hyopneumoniae P102 protein and applications thereof
InactiveCN101659954AStimulate immune protectionImprove stabilityAntibacterial agentsGenetic material ingredientsBALB/cBacteroides
The invention discloses a recombinant adenovirus expressing mycoplasma hyopneumoniae P102 protein and applications thereof. The recombinant adenovirus is obtained by firstly carrying out mutation on gene of the mycoplasma hyopneumoniae P102 protein, cloning the whole genome (SEQ ID No:1) after the mutation onto a shuttle vector pShuttle-CMV, obtaining a recombinant shuttle vector, carrying out electro-transformation into competent cells containing an adenovirus backbone vector after linearization and carrying out homologous recombination in bacteria. The indirect immunofluorescence and the Western Blot identification prove that the recombinant adenovirus successfully expresses the P102 protein. The recombinant adenovirus is used for immunizing female Balb / c mice with 6-8 weeks by two waysof muscle injection and nasal dropping, and the recombinant virus can induce the mice to produce the specific humoral immune and the cellular immune response, thereby indicating that the recombinant adenovirus can be applied in the diagnosis, the prevention or the treatment of mycoplasma pneumoniae of swine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Traditional Chinese medicine compound preparation for treating swine mycoplasmal pneumonia and preparation method thereof
ActiveCN105031025ASimple production processConvenient clinical administrationAntibacterial agentsRespiratory disorderDrug contentFlos chrysanthemi
The invention belongs to a traditional Chinese medicine compound preparation for treating swine mycoplasmal pneumonia and a preparation method thereof. The traditional Chinese medicine compound preparation is composed of, by weight, 10-20 parts of radix saposhnikoviae, 5-15 parts of radix astragali, 5-10 parts of radix asteris, 10-15 parts of tussilago farfara, 10-15 parts of radix scrophulariae, 5-15 parts of coxtex moutan, 5-10 parts of radix glycyrrhizae, 10-15 parts of radix lithospermi, 10-20 parts of mulberry leaves and 10-20 parts of flos chrysanthemi. The traditional Chinese medicine compound preparation for treating the swine mycoplasmal pneumonia is rich in raw material source, simple in production technology, easy to absorb after entering a body, convenient to administrate in clinic and suitable for being administrated in groups, and labor and material sources are saved; according to the traditional Chinese medicine compound preparation, the monarch, minister, assistant and guide compatibility principle of the traditional Chinese medicine is adopted, the medicine content is clear, medicine administration is accurate, the quality is controllable, traditional Chinese medicine treatment is performed for the swine mycoplasmal pneumonia, and the medicine is safe, effective and free of residues and does not have the medicine resistance.
Owner:河南省帝一方生物制药有限公司
Veterinary herbal composition for preventing and treating swine mycoplasmal pneumonia and preparation method of veterinary herbal composition
InactiveCN110279796AFacilitate the treatment of infectionPromote barrier permeabilityAntibacterial agentsHydroxy compound active ingredientsSemenSodium sulfate
The invention relates to a veterinary herbal composition for preventing and treating swine mycoplasmal pneumonia and a preparation method of the veterinary herbal composition. The veterinary herbal composition is prepared from the following components in parts by weight: 10 to 30 parts of radix glycyrrhizae, 14 to 34 parts of radix isatidis, 4 to 20 parts of pulvis fellis suis, 5 to 15 parts of bulbus fritillariae thunbergii, 5 to 15 parts of radix rehmanniae, 5 to 15 parts of fructus perillae, 4 to 12 parts of semen armeniacae amarae, 3 to 11 parts of radix scutellariae, 2 to 8 parts of weathered sodium sulfate and 1 to 7 parts of borneolum syntheticum. The preparation method comprises the following steps: adding the radix glycyrrhizae, the radix isatidis, the bulbus fritillariae thunbergii, the radix rehmanniae, the fructus perillae, the semen armeniacae amarae and the radix scutellariae into water of which the amount is 12 times that of medicinal materials, and decocting for three times, with two hours each time; combining decocted liquid, filtering, and carrying out vacuum concentration and spray drying on filtrate for later use; then taking the pulvis fellis suis, the weathered sodium sulfate and the borneolum syntheticum, crushing, sieving and uniformly mixing a sieved material with an extract obtained by spray drying to obtain a finished product. The preparation method disclosed by the invention has the advantages of simple steps and stable process; the veterinary herbal composition can be used for clearing heat, removing toxicity and relieving cough and asthma, and has relatively high safety and effectiveness for preventing and treating the swine mycoplasmal pneumonia.
Owner:BEIJING CENT BIOLOGY
Feed for preventing swine mycoplasmal pneumonia
InactiveCN106901020APrevent pneumoniaImprove immunityAntibacterial agentsAnthropod material medical ingredientsAnimal scienceFlos chrysanthemi
The invention relates to feed for preventing swine mycoplasmal pneumonia. The feed is characterized by being prepared from the following raw materials in parts by weight: 450-480 parts of corn, 120-140 parts of broken rice, 120-150 parts of soybean meal, 50-70 parts of germ meal, 120-140 parts of cassava flour, 10-20 parts of zeolite powder, 5-10 parts of bone meal, 5-10 parts of garlic powder, 6-10 parts of herba ephedra, 3-6 parts of semen armeniacae amarum, 8-12 parts of rhizoma polygontum cuspidatum, 10-15 parts of radix bupleuri, 8-12 parts of sweet wormwood herb, 5-8 parts of lumbricus, 6-10 parts of flos chrysanthemi indici and 5-8 parts of bombyx batryticatus. The feed provided by the invention has a good effect of preventing the swine mycoplasmal pneumonia, and is helpful in enhancing immunity and promoting growth and development.
Owner:CHANGSHA RUIDUOKANG BIOTECH CO LTD
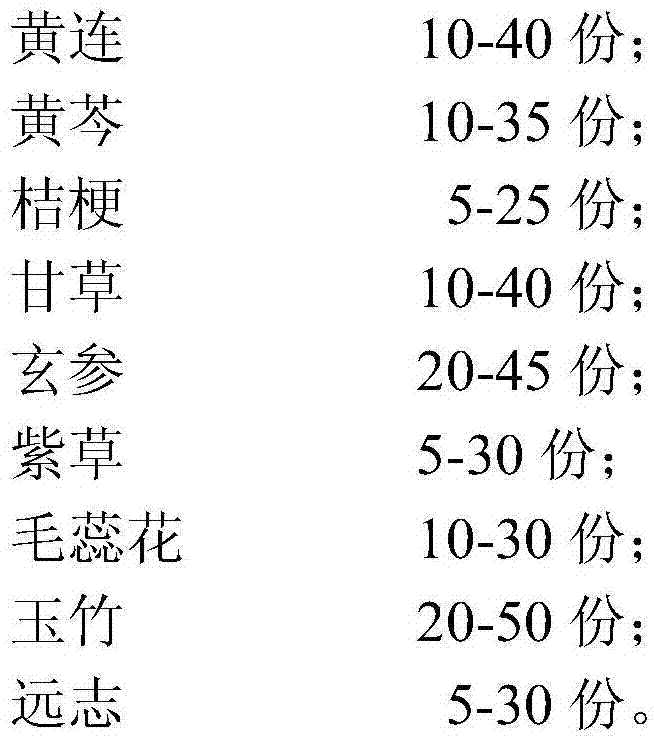
Traditional Chinese medicine composition for treatment of swine mycoplasmal pneumonia and preparation method of traditional Chinese medicine composition
InactiveCN106924531AEffective treatmentMoisturizing the lungs and relieving coughAntibacterial agentsRespiratory disorderOfficinalisLiquorices
The invention relates to a traditional Chinese medicine composition for treatment of swine mycoplasmal pneumonia. The traditional Chinese medicine composition comprises, by weight, 10-40 parts of coptis chinensis, 10-35 parts of radix scutellariae, 5-25 parts of platycodon grandiflorum, 10-40 parts of liquorice, 20-45 parts of radix scrophulariae, 5-30 parts of radix arnebiae seu lithospermi, 10-30 parts of flores verbasci, 10-50 parts of radix polygonati officinalis and 5-30 parts of polygala tenuifolia. The traditional Chinese medicine composition is scientific and reasonable in formulation, convenient in use and low in cost, and the treatment rate of the swine mycoplasmal pneumonia is around 90%.
Owner:TIANJIN ZHONGAO BIOTECH
Additive for preventing and treating swine mycoplasma pneumonia and preparation method and application thereof
ActiveCN112494594AReduce mucusLess waxyAntibacterial agentsFood processingBiotechnologyRadix Astragali seu Hedysari
The invention discloses an additive for preventing and treating swine mycoplasma pneumonia and a preparation method and application thereof, and belongs to the technical field of feed additives. The additive is prepared from the following components in parts by mass: 100 to 300 parts of honeysuckle flower, 100 to 200 parts of radix scutellariae, 200 to 300 parts of radix astragali seu hedysari, 200 to 300 parts of radix platycodonis, 100 to 300 parts of dried tangerine peel, 100 to 200 parts of apricot kernel, 100 to 200 parts of rhizoma atractylodis, 100 to 200 parts of bulbus fritillariae ussuriensis and 50 to 100 parts of liquorice root. Compared with the prior art, the additive disclosed by the invention has the beneficial effects that the feed additive is produced by utilizing ultrafine powder natural plants; cell walls of the plants can be damaged through crushing, and effective components of the plants are sufficiently released; and the additive has an inhibition effect on pathogens including mycoplasma, viruses, bacteria and the like, and also has the effects of removing heat, relieving cough and reducing phlegm, relieving asthma and enhancing the immunity of pigs. The feedadditive is prepared from pure natural plants, is safe and effective, has low toxic and side effects and has no residues.
Owner:ANIMAL HUSBANDRY RES INST OF HEILONGJIANG ACADEMY OF AGRI SCI
Production method of swine mycoplasmal pneumonia inactivated vaccine
The invention relates to a production method of a swine mycoplasmal pneumonia inactivated vaccine. The swine mycoplasmal pneumonia inactivated vaccine disclosed by the invention is produced by successfully separating a mycoplasma hyopneumoniae S strain having good immunogenicity and used as a production bacterial strain, and researching a proper cultural method, an inactivating process and a proper immunologic adjuvant by using a high-efficiency culture medium. After being used for immunized piglets, the inactivated vaccine produced by the invention can obtain good immune protective efficacy.
Owner:北京中海生物科技有限公司 +2
Composition and preparation method for treating swine mycoplasma and porcine pleuropneumonia
ActiveCN105147802BGood treatment effectEasy to prepareAntibacterial agentsAnthropod material medical ingredientsVeterinary DrugsTherapeutic effect
The invention relates to the field of veterinary drugs, in particulrl to a composition for treating swine mycoplasmal pneumonia and porcine contagious pleuropneumonia and a preparation method thereof. The composition is prepared from, by weight, 15-20 parts of rhizoma atractylodis, 15-20 parts of radix saposhnikoviae, 10-13 parts of liquorice, 10-15 parts of apricot kernels, 30-35 parts of radix astragali, 15-20 parts of radix asteris, 10-15 parts of flos farfarae, 10-15 parts of herba ephedrae, 10-15 parts of periostracum cicada, 10-15 parts of fructus forsythiae and 20-30 parts of lonicera japonica. The composition has the effects of clearing heat, discharging fire, diminishing inflammation, resisting bacteria and the like, all the traditional Chinese medicines are proportioned reasonably to complement and coordinate mutually, the good treatment effect on the swine mycoplasmal pneumonia and the porcine contagious pleuropneumonia is achieved, the treatment course is short, the effects are rapid to achieve, and the cure rate is high; meanwhile, the preparation method of the composition is simple, safe, reliable, free of pollution and residues and easy to popularize.
Owner:DALIAN NATIONALITIES UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com