Bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma hyopneumoniae and preparation method of bigeminy inactivated vaccine
A technology of mycoplasma hyopneumoniae and dual inactivated vaccines, which is applied in the fields of virus antigen components, antiviral agents, and pharmaceutical formulations, can solve the problem of no porcine circovirus mycoplasma hyopneumoniae dual combination vaccine, etc., and achieve a long duration of immunity , less time-consuming, and cost-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
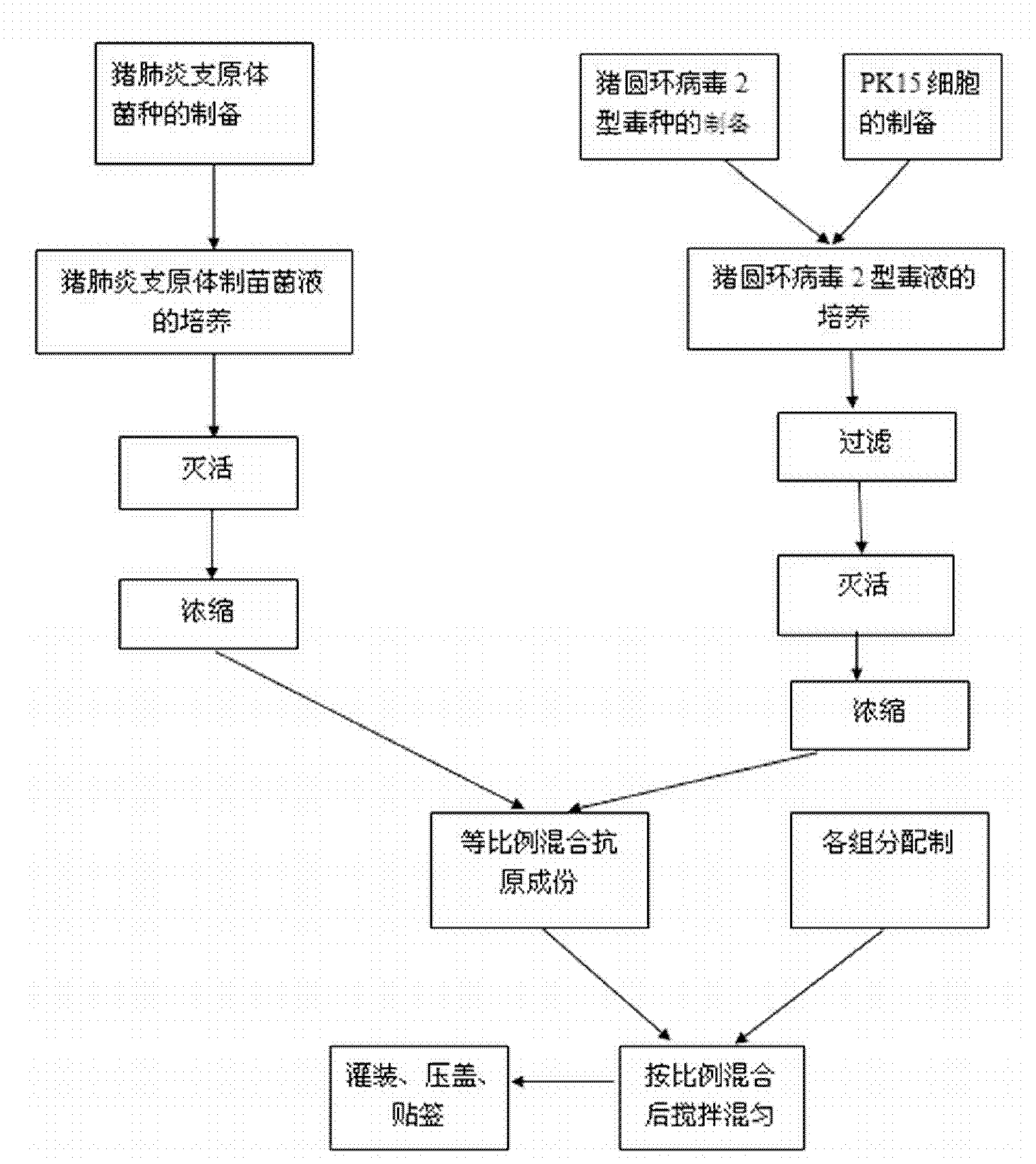
[0028] Embodiment 1, the preparation of porcine circular ring, mycoplasma pneumoniae dual combination inactivated vaccine
[0029] 1. The source of bacteria (virus) strains
[0030] The selected porcine circovirus strain is the PCV-2b strain SH strain, which has been preserved in the General Microbiology Center of the China Microbiological Culture Collection Management Committee. The preservation date: March 4, 2008, and the preservation number is CGMCC No.23890 .
[0031] The selected strain of Mycoplasma hyopneumoniae was CVCC355, which was purchased from China Veterinary Drug Control Institute.
[0032] 2. Preparation and inspection of vaccine semi-finished products
[0033] 2.1 Preparation of seeds for production
[0034] 2.1.1 Preparation of porcine circovirus type 2: Dilute the virus seeds appropriately with breastmilk liquid, inoculate PK15 cells (purchased from China Veterinary Drug Control Institute) at 5% for culture, absorb at 37°C for 30 minutes, add 4% Calf seru...
Embodiment 2
[0076] Example 2, the comparison of the immune protection effect and the serological effect of the dual inactivated vaccine product trial-produced in Example 1 and two kinds of monovalent inactivated vaccines (mycoplasma hyopneumoniae inactivated vaccine or porcine circovirus type 2 inactivated vaccine) evaluate
[0077] In 1 material embodiment 1, the dual inactivated vaccine trial product of porcine circovirus type 2 and mycoplasma hyopneumoniae; porcine circovirus type 2 inactivated vaccine (SH strain), produced by Pulaike Bioengineering Co., Ltd. (batch number 100903); American Schering-Plough Inactivated Vaccine for Mycoplasma Porcine Pneumonia (J strain) (batch number 100806).
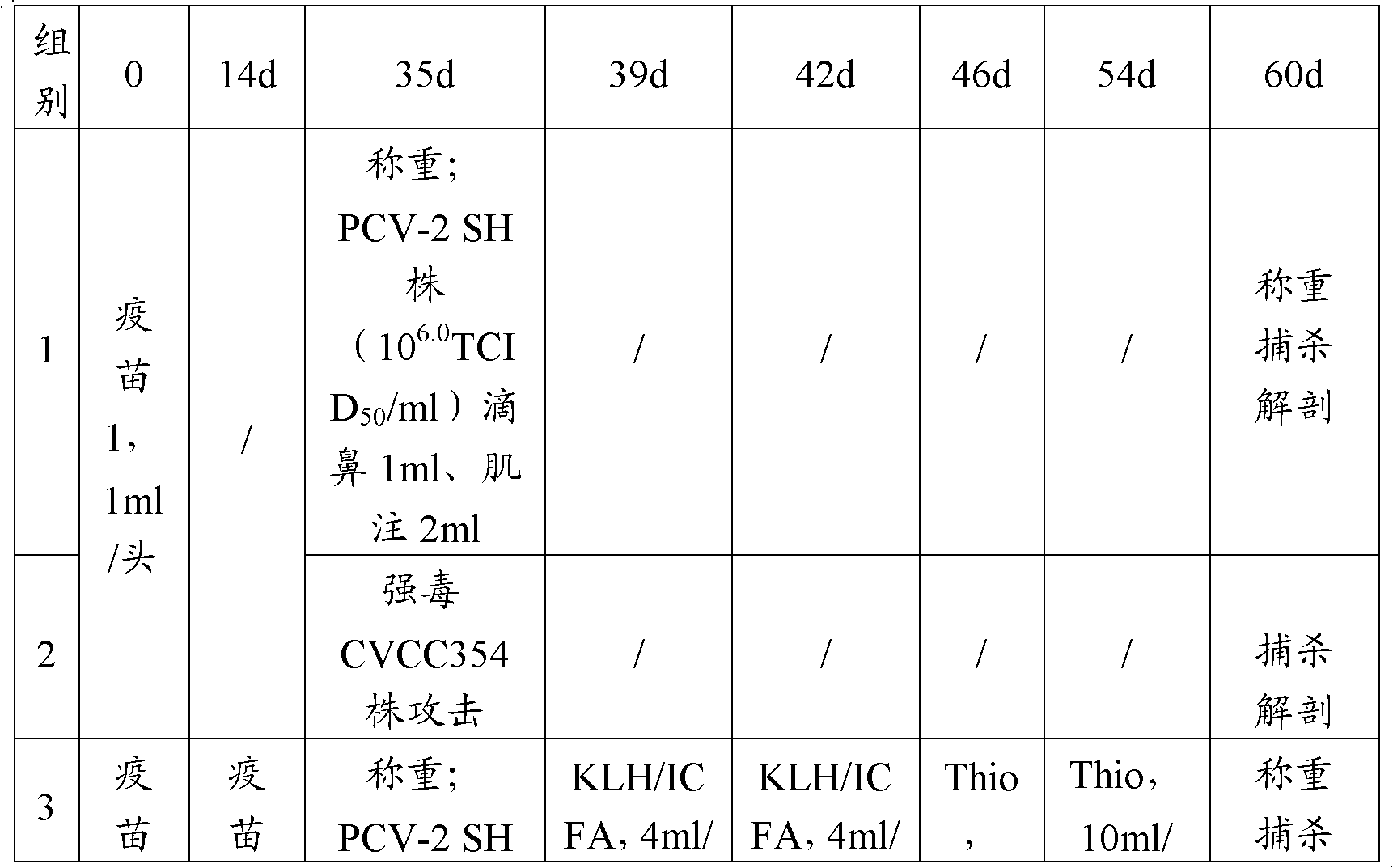
[0078] 2 Animal experiment design
[0079]Select 70 weaned piglets aged 21-28 days and divide them into 7 groups, 10 pigs in each group (see the table below); each pig in the 1st and 2nd groups was intramuscularly injected with porcine ring and Mycoplasma pneumoniae dual inactivated vaccine 1ml...
Embodiment 3
[0110] Research on the duration of immunity of trial-produced products in Example 3 and Example 1
[0111] In 1 material embodiment 1, the dual inactivated vaccine trial product of porcine circovirus type 2 and mycoplasma hyopneumoniae; porcine circovirus type 2 inactivated vaccine (SH strain), produced by Pulaike Bioengineering Co., Ltd. (batch number 100903); American Schering-Plough Inactivated Vaccine for Mycoplasma Porcine Pneumonia (J Strain) (Lot No. 100806)
[0112] 2 Animal experiment design
[0113] Select 70 weaned piglets aged 21 to 28 days and divide them into 7 groups with 10 piglets in each group. See the table below for the grouping treatment:
[0114]
[0115]
[0116] Note:
[0117] A, vaccine 1 is porcine circovirus type 2 inactivated vaccine trial product in embodiment 1, Mycoplasma pneumoniae dual inactivated vaccine; Vaccine 2 is porcine circovirus type 2 inactivated vaccine (SH strain), produced by Pulaike Bioengineering Co., Ltd. ( The batch nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com