Long-acting animal rabies vaccine and preparing method thereof
A technology of rabies vaccine and rabies virus, which is applied in the field of long-acting rabies vaccine for animals and its preparation, can solve the problems of short duration and achieve the effects of safe use, wide range of animals, and long duration of immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Construction of rabies virus glycoprotein-recombinant retroviral vector
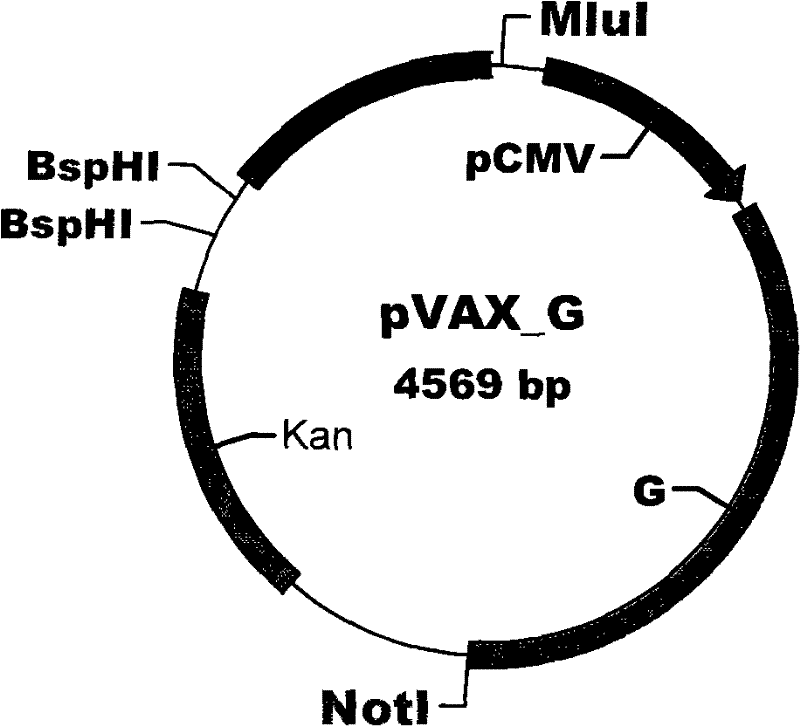
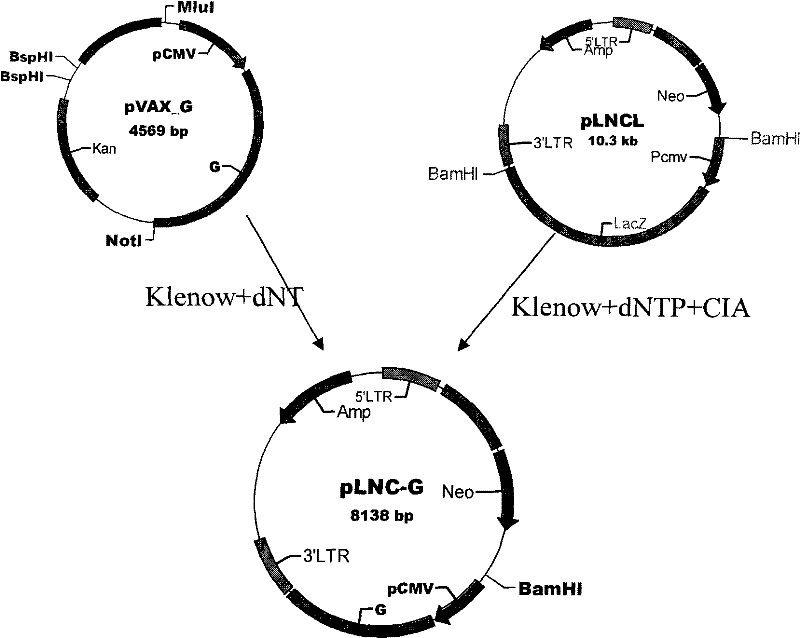
[0029] The pLNCL vector containing the lacZ gene and the plasmid vector pVAX-G were constructed and preserved by our laboratory; the packaging cell line PA 317, Escherichia coli (E.coli) JM109, was purchased commercially and preserved by our laboratory.
[0030] The main process of recombinant plasmid construction is as follows: (1) After BamH I digests pLNCL, the linearized fragment of 5812bp is recovered, filled with Klenow enzyme, and then dephosphorylated by alkaline phosphatase (CIAP) to obtain both ends Both are blunt-ended pLNCX empty vectors. (2) BspHI, Not I and Mlu I three enzymes are combined to digest pVAX-G, reclaim the 2326bp fragment, and fill it up with Klenow enzyme, so as to obtain a nucleic acid with both ends being blunt and containing the G protein gene of rabies virus SRV9 strain fragment. (3) The 2326bp nucleic acid fragment containing the G protein gene of the rabies viru...
Embodiment 2
[0073] Packaging of recombinant virus
[0074] The minimum lethal dose of G418 to the packaging cell line PA 317 was determined with different concentrations of G418 ranging from 0.2 to 0.7 g / L. Referring to the instructions of Lipofectamine 2000, the recombinant virus vector was introduced into the packaging cell line PA 317 with liposome as the medium. And screened with the least lethal dose of G418.
[0075] (1) Determination of the minimum lethal dose of G418 by PA317 and its results
[0076] Inoculate an appropriate amount of PA317 cells in a 24-well plate, and when the cells grow into 75% confluent pieces, add selection medium containing different concentrations of G418 (0.2, 0.3, 0.35, 0.4, 0.5, 0.6, 0.7g / L), each gradient Three repetitions were set up, and a blank control was set at the same time. The medium was changed every 3 days, and the minimum lethal concentration of G418 for PA317 cells was determined according to the cell death situation in 10-14 days. On t...
Embodiment 3
[0085] Cloning of Toxigenic Cells and Expansion of Recombinant Viruses
[0086] Add feeder cells to each well of the 96-well plate, mark the position of the clone with a marker pen on the 12-well plate where cell clones grow, pick the cell clone with a sterile tip on the ultra-clean workbench, and inject it into the 96-well plate After the cells adhere to the wall, replace half of the liquid in the 96-well plate with DMEM nutrient solution with a final concentration of 400 μg / L G418, and then replace all the liquid with DMEM nutrient solution with a final concentration of 400 μg / L G418 after the cells grow Carry out culture, after overgrown, transfer into 24-well plate, 6-well plate, 30ml cell culture bottle, 50ml cell culture bottle successively, expand culture gradually. When expanding the culture, the concentration of G418 was 400 μg / ml, and the amount of serum added was 10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com