A kind of human rabies vaccine and preparation method thereof
A technology of rabies vaccine and rabies virus, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, antiviral agents, etc., can solve the problems of long immune response time, low level of persistent immunity, and inability to quickly treat diseases. The effect of long duration, easy availability of equipment, and improvement of immune response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
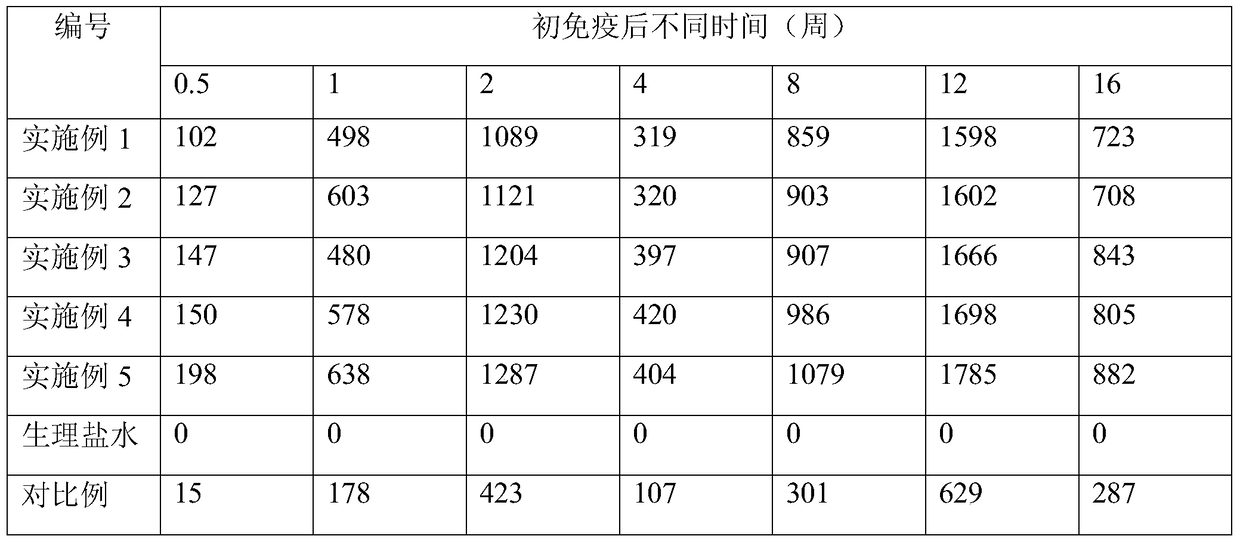
Embodiment 1
[0026] The human rabies vaccine consists of the following components: 0.1 mg of zinc hydroxide, 10 μg of lanolin, 10 μg of bacterial lipopolysaccharide and 10 μg of Tween-80 are used as raw materials for each dose of the inactivated rabies virus vaccine.
[0027] Preparation method: Add zinc gluconate into sterile double-distilled water, fully stir and dissolve to obtain zinc gluconate solution. Sodium hydroxide was added into sterile double-distilled water, fully stirred and dissolved to obtain a sodium hydroxide solution. Under sterile conditions, the zinc gluconate solution and the sodium hydroxide solution were filtered and sterilized with a closed membrane filter respectively, and then used for later use. Under medium-speed stirring, slowly drop the sodium hydroxide solution into the zinc gluconate solution, and stir thoroughly to obtain the zinc hydroxide colloid, which is set aside;
[0028] Add lanolin and bacterial lipopolysaccharide respectively into sterile double-...
Embodiment 2
[0031] The human rabies vaccine consists of the following components: 1 mg of zinc hydroxide, 100 μg of lanolin, 100 μg of bacterial lipopolysaccharide, and 100 μg of Tween-80 are used as raw materials for each dose of the inactivated rabies virus vaccine.
[0032] Preparation method: same as Example 1.
Embodiment 3
[0034] The human rabies vaccine consists of the following components: 2 mg of zinc hydroxide, 300 μg of lanolin, 300 μg of bacterial lipopolysaccharide, and 300 μg of Tween-80 are used as raw materials for each dose of the inactivated rabies virus vaccine.
[0035] Preparation method: same as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com