A kind of bivalent inactivated vaccine of bovine pasteurellosis multocida and preparation method thereof
A technology of bivalent inactivated vaccine and Pasteurella, which can be used in antibacterial drugs, blood diseases, and pharmaceutical formulations, and can solve the problem of poor cross-immunity and the inability to effectively prevent and control bovine fibrinous suppurative pneumonia and bovine multocida Pasteurellosis is difficult to prevent and control, to achieve the effect of reducing side effects, good immune protection effect, and reducing the content of bacterial exotoxins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
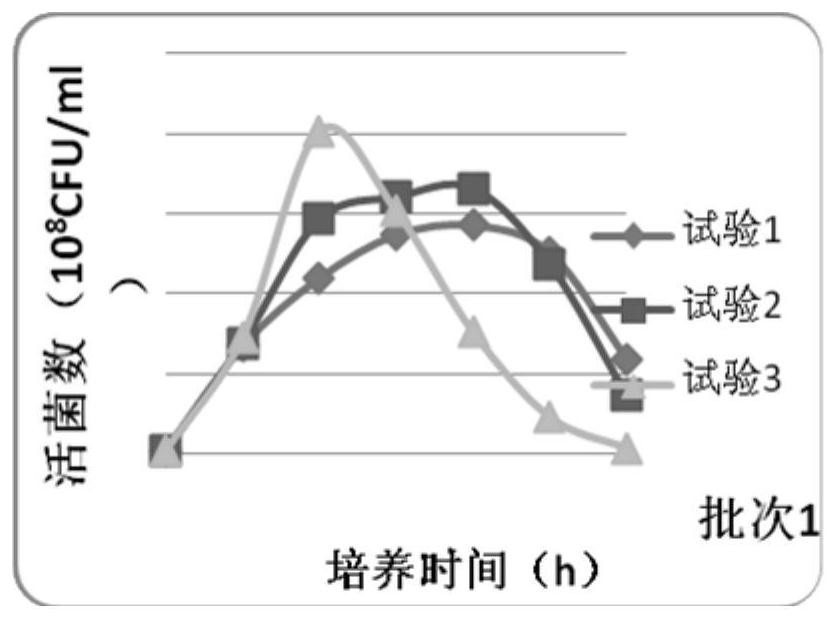
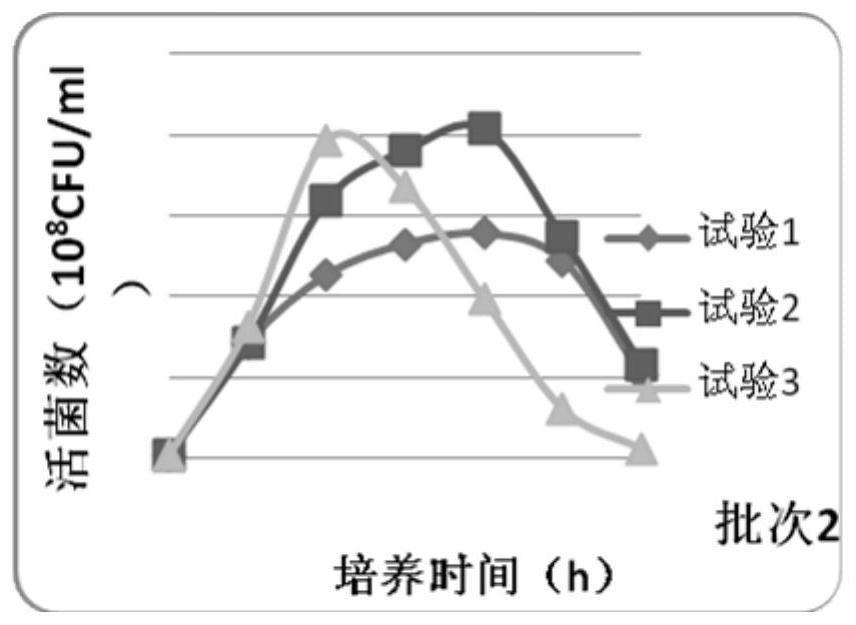
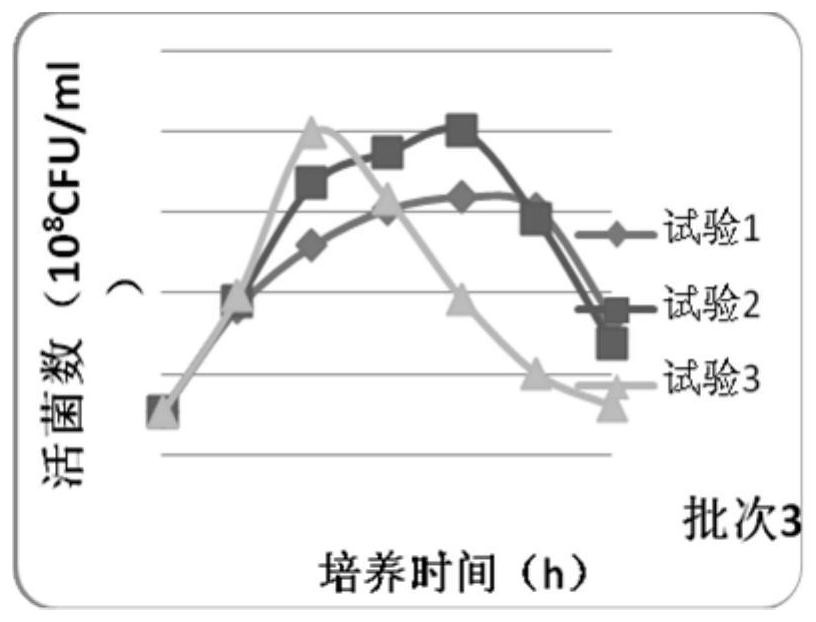
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Bivalent Inactivated Vaccine for Bovine Pasteurella Multocida
[0031] 1. Medium
[0032] Preparation of Brain Heart Infusion Medium (BHI): Weigh 37g of BHI powder and add it to 1000ml of distilled water, mix thoroughly, and then sterilize at 121°C for 15 minutes.
[0033] Preparation of Martin Broth Medium: Weigh 50g of Martin Broth Medium Powder and add it to 1000ml of distilled water. After mixing thoroughly, sterilize at 121°C for 15 minutes. After sterilization, add 0.1% lysed hemocyte whole blood according to the volume of the medium.
[0034] Brain Heart Infusion Agar Medium Preparation: Weigh 37g of BHI powder and 15g of agar powder into 1000ml of distilled water, mix thoroughly, sterilize at 121°C for 15 minutes, cool to 50°C to 60°C, pour the plates for later use.
[0035] Preparation of Martin Broth Agar Medium: Weigh 50g of Martin Broth Medium Powder and 15g of Agar Powder into 1000ml of distilled water, mix well, sterilize at 121°C...
Embodiment 2
[0055] Example 2 The safety test of the vaccine prepared by the present invention
[0056] 1 material
[0057] The vaccine that the method for 1.1 embodiment 1 makes bovine pasteurellosis multocida bivalent inactivated vaccine (A type Pm-TJ strain+B type C45-2 strain). The batch numbers are ZM201401, ZM201402, ZM201403 respectively.
[0058] 1.2 Experimental animals Healthy susceptible cattle, 3 months old and above.
[0059] 2 methods
[0060] 2.1 Safety test of a single-dose inoculation of target animals
[0061] 12 test cows were randomly divided into four groups, and 3 batches of vaccines were inoculated through the neck muscles with a single dose of 2.0ml (1 head), and 3 test cows with the same conditions were set up. Cattle were inoculated with sterilized PBS as a control in the same way. The clinical manifestations of the test cattle were observed, and the body temperature was measured at the same time every day for 14 days.
[0062] 2.2 Safety test of single-dose...
Embodiment 3
[0082] Example 3 Efficacy Test of the Vaccine Prepared by the Present Invention
[0083] 1 material
[0084] 1.1 The vaccine made by the method of Example 1 Bovine Pasteurella multocida bivalent inactivated vaccine (A type Pm-TJ strain+B type C45-2 strain). The batch numbers are ZM201401, ZM201402, ZM201403 respectively. The batch number of inactivated bovine pasteurellosis multocida vaccine (type B strain single vaccine, C45-2 strain, C46-2 strain, and C47-2 strain all contains) is 1407001.
[0085] 1.2 The experimental animals are healthy and susceptible cattle, aged 6 months and above.
[0086] 2 methods
[0087] 3 batches of vaccines prepared by the present invention were inoculated into 8 test cows with 1 part (2.0ml) dose each muscle, and the commercially available bovine pasteurellosis multocida inactivated vaccine (type B bacteria single vaccine) was injected with 1 cow. Each dose (4.0ml) was intramuscularly inoculated to 4 test cows, and another 6 non-immunized co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com