Swine Mycoplasmal pneumonia live vaccine mucosal immune adjuvant, preparation method and application thereof
A technology of mycoplasma pneumonia and immune adjuvant, which is applied in vaccines, veterinary vaccines, chemical instruments and methods, etc., can solve the problem of the effect of adjuvant components, unclear action principle, lack of mucosal immune adjuvant, difficulty in adjuvant selection, etc. To improve the efficacy of mucosal immune protection, prolong the duration of immunity, and achieve the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction of recombinant engineering bacteria BL21-pET28a-CTB-P97R1
[0033]In order to screen the mucosal immune adjuvant of the live vaccine against Mycoplasma pneumoniae in swine, a variety of fusion proteins were designed. After testing, only the fusion protein CTB-P97R1 was found to have a better immune effect. The amino acid sequence of the fusion protein CTB-P97R1 is shown in SEQ ID NO:2, and the gene sequence is shown in SEQ ID NO:1.
[0034] This example describes the construction process of the recombinant genetically engineered bacteria BL21-pET28a-CTB-P97R1 used to prepare the fusion protein CTB-P97R1.
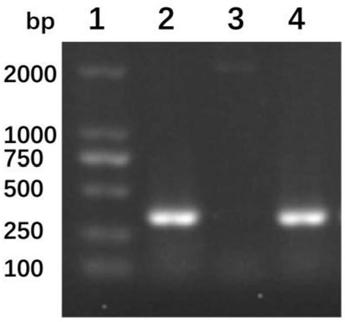
[0035] According to the gene sequence (AY307389.1) of cholera toxin B subunit (CTB) included in GenBank, the CTB gene (see SEQ ID NO: 3 for the specific sequence) was obtained by gene synthesis, and at both ends Add PagI and NheI restriction sites, respectively. The above synthetic sequence was double digested and purified, and the pET-28a(+...
Embodiment 2
[0037] Example 2: Expression and purification of fusion protein CTB-P97R1
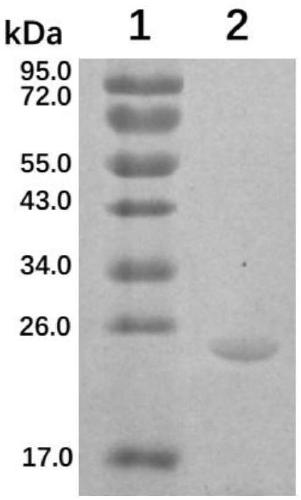
[0038] The positive recombinant bacterium BL21-pET28a-CTB-P97R1 was cultured overnight at 37° C. with shaking in LB medium (kanamycin resistant). The next day, the bacterial solution was inoculated in LB (kanamycin-resistant) liquid medium at a ratio of 2%, and cultured with shaking at 37°C until OD 600 =0.4-0.6, adding isopropyl-β-D-thiogalactopyranoside (IPTG) with a final concentration of 1.0mmol / L to induce expression, collect the bacterial liquid 4 hours after induction, centrifuge to get bacterial precipitate, wash with PBS 1 time. The cells were suspended in PBS buffer, ultrasonically lysed, centrifuged at 10,000 rpm for 30 min at 4°C, and the lysed supernatant was separated and purified by His-Tag affinity chromatography column, dialyzed for 2 days to remove salt, and freeze-dried. The purity of fusion protein CTB-P97R1 was analyzed by 15% SDS-PAGE electrophoresis. From figure 2 It can be ...
Embodiment 3
[0039] Example 3 In vitro biological activity detection of fusion protein CTB-P97R1
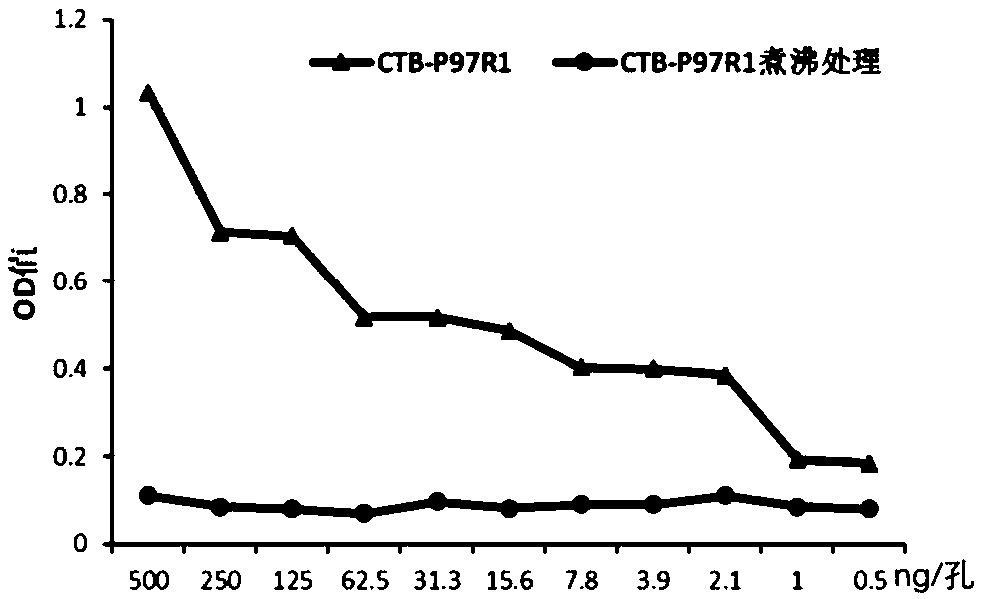
[0040] Methods: Ganglioside GM1-enzyme-linked immunosorbent assay (ELISA) was used to detect the binding ability of fusion protein CTB-P97R1 and ganglioside GM1. Carbonate buffer containing 5 μg / ml GM1 (containing 35 mmol / L NaHCO 3 , 15mmol / L Na 2 CO 3 aqueous solution) at 4°C overnight, 100 μl per well; washed 3 times with PBST (PBS buffer containing 0.1% Tween-20), blocked with PBS solution containing 1% BSA at 37°C for 2 hours, washed 3 times with PBST, Add 100 μl of a solution containing 500 ng of CTB-P97R1 (purified) to each well, incubate at 37° C. for 2 hours, and replace it with CTB-P97R1 solution boiled at 100° C. for 10 minutes in the control. After washing with PBST, rabbit anti-CTB antibody (Bio-Rad, USA) diluted with PBS at a dilution ratio of 1:4000 was added, and incubated at 37°C for 1 h. After washing with PBST for 3 times, horseradish peroxidase-labeled goat anti-rabbit ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com