Porcine reproductive and respiratory syndrome, swine mycoplasmal pneumonia combined live vaccine and preparation method thereof
A technology for swine mycoplasma pneumonia and respiratory syndrome, which is applied in the direction of pharmaceutical formulations, bacterial antigenic components, viral antigenic components, etc., can solve the problems of decreased immune effect, animal stress and safety issues, and achieve simple preparation methods and strengthen mutual immunity Effect, the effect of high vaccine titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 mycoplasma pneumonia live vaccine (168 strains) antigen and porcine reproductive and respiratory syndrome live vaccine (R98 strain) antigen
[0040] 1. Antigen Preparation of Live Vaccine of Mycoplasma Porcine Pneumonia (168 strains)
[0041] 1.1 Source of the strain:
[0042] The 168 strains of Mycoplasma hyopneumoniae used in the manufacture and testing of this product were provided by Guizhou First Biotechnology Co., Ltd.
[0043]1.2 Preparation of seeds for production Take freeze-dried strains and inoculate them in KM at a volume ratio of 1:100-1000 2 In the culture medium, culture at 37°C for about 3 to 7 days. When the culture changes color and is slightly turbid, take a culture smear for Wright staining and microscopic examination. When the bacterial morphology meets the standard and the pure inspection is qualified, expand the culture. . The number of succession does not exceed 5 generations.
[0044] 1.3 Medium KM for making ...
Embodiment 2
[0059] The preparation of embodiment 2 porcine reproductive and respiratory syndrome (R98 strain), swine mycoplasma pneumonia (168 strain) dual live vaccine
[0060] 1. Vaccine lyoprotectant treatment
[0061] Sucrose-gelatin adjuvant: Dissolve 1.5% gelatin and 12.5% sucrose in water, autoclave at 115°C for 30 minutes, adjust the pH to 7.0 with sterile NaOH, and place in a greenhouse at 37°C for later use.
[0062] 2. Matching seedlings
[0063] Porcine reproductive and respiratory syndrome (R98 strain) antigen and porcine mycoplasma pneumonia (168 strain) antigen were mixed in a ratio of 1:1 by aseptic operation, then added with the same volume of sucrose gelatin adjuvant, and mixed evenly. Make porcine reproductive and respiratory syndrome (R98 strain) antigen content ≥ 10 5.0 TCID 50 / head, mycoplasma pneumonia (168 strains) antigen content ≥ 10 6.0 CCU / Touquan.
[0064] 3. Subpackaging and freeze-drying
[0065] The uniformly mixed vaccine is prepared and the spec...
Embodiment 3
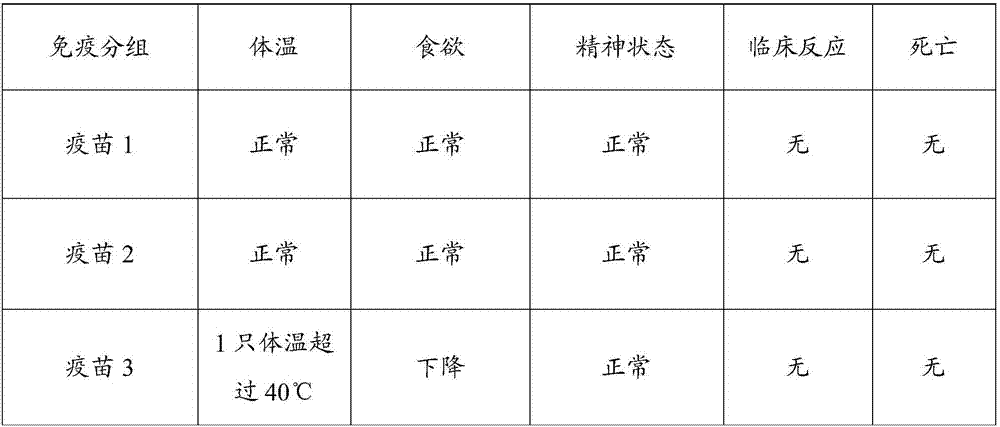
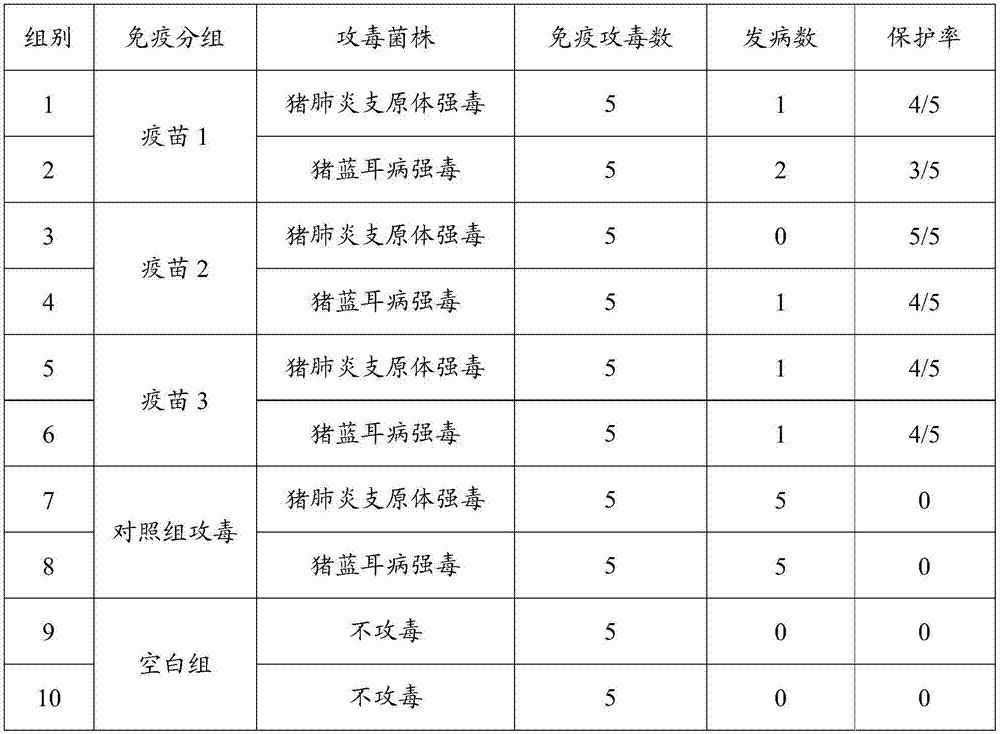
[0067] Example 3 Different antigen content porcine reproductive and respiratory syndrome (R98 strain), swine mycoplasma pneumonia (168 strain) dual live vaccine efficacy test
[0068] 1. Test material
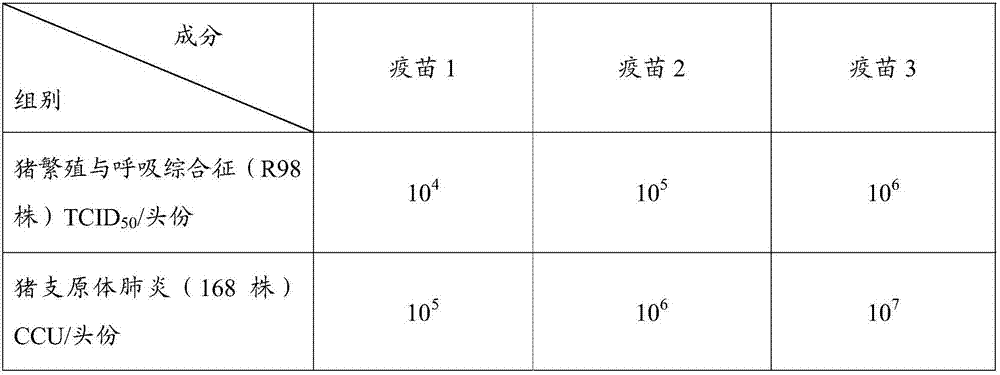
[0069] Have been divided into groups in embodiment 2, vaccine 1 (porcine reproductive and respiratory syndrome (R98 strain) 10 4 TCID 50 / Toufen + Mycoplasma pneumoniae (168 strains) 10 5 CCU / head portion), vaccine 2 (porcine reproductive and respiratory syndrome (R98 strain) 10 5 TCID 50 / Toufen + Mycoplasma pneumoniae (168 strains) 10 6 CCU / head portion) and vaccine 3 (porcine reproductive and respiratory syndrome (R98 strain) 10 6 TCID 50 / Toufen + Mycoplasma pneumoniae (168 strains) 10 7 CCU / Toufen)
[0070] 1-2 week-old Bama pigs free of Mycoplasma hyopneumoniae and PRRS antibodies and antigens.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com