Recombinant protein of Mycoplasma pneumonia and porcine circovirus type 2 and bivalent vaccine prepared therewith
A recombinant protein, vaccine technology, applied in the field of vaccines, can solve problems such as increasing mortality, increasing disease severity and potential persistence, and economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
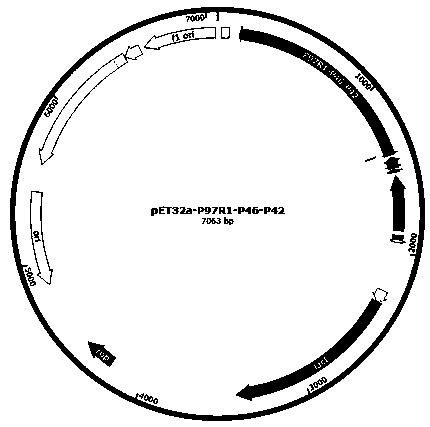
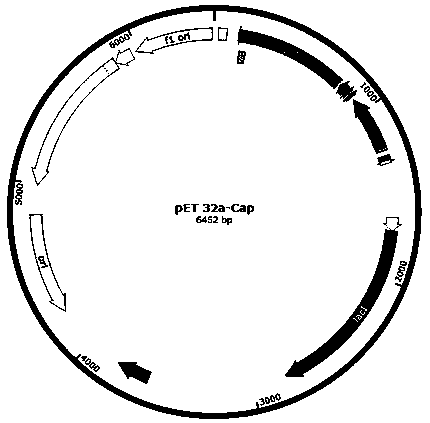
[0052] Acquisition of target gene and construction of expression vector
[0053] Search the Shandong strain PCV2 Cap gene sequence (accession number: KY656098.1) in the NCBI database to remove the nuclear localization signal peptide, and optimize the codons of the prokaryotic expression system for the remaining nucleic acid sequence. The same method was used to search for the P97R1 (MHP168_110), P46 (MHP168_522) and P42 (MHP168_069) gene sequences of the Mhp168 strain (accession number: CP002274.1), and optimize the codons. Combined with the pET32a expression vector multiple cloning site and gene sequence to select the appropriate restriction site and connecting peptide sequence, the following two sequences were chemically synthesized: ①NcoI-P97R1-GGSG-P46-GGSG-P42-XhoI; ②KpnI-Cap-XhoI.
[0054] Using molecular biology methods, the two sequences synthesized above were double-digested and ligated into the pET32a vector, and then transformed into TG1 cloning bacteria. The recomb...
Embodiment 2
[0057] Expression and purification of target protein
[0058] The pET32a-P97R1-P46-P42 and pET32a-Cap were transformed into BL21 DE3 expression bacteria, respectively, for induced expression and purification. The methods of obtaining and operating the two proteins are the same, specifically as follows:
[0059] ①Protein expression: insert recombinant expression bacteria into LB medium at a ratio of 1:100, add antibiotics with a final concentration of 1 mM Amp, shake the bacteria at 37°C and 220 rpm for 3-4 h, and then add a final concentration of 1 mM IPTG to induce , 16°C, 220 rpm to induce overnight culture.
[0060] ② Bacterial cell crushing: collect the cultured bacterial liquid induced overnight, and centrifuge at 4000 rpm for 40 min to obtain the bacterial cell; resuspend and wash with an appropriate amount of sterile PBS solution, centrifuge at 12000 rpm, 4°C for 10 min, and discard the supernatant; use a small amount of pre-cooled The bacterial cells were resuspended...
Embodiment 3
[0063] Identification, concentration and quantitative analysis of target protein
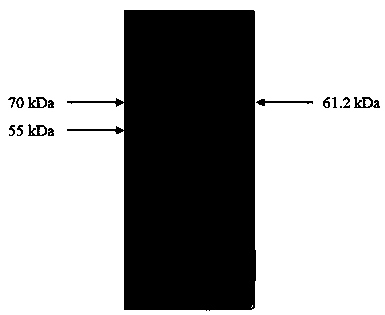
[0064] After the purified P97-P46-P42 and Cap were prepared separately, they were electrophoresed on 12% SDS-PAGE gel, stained with Coomassie brilliant blue, and decolorized. The results are as follows image 3 with Figure 4 . Using His antibody for Western Blot identification, the results are as follows Figure 5 with Image 6 .
[0065] On the basis of the above identification, the two proteins were concentrated and desalted using 10 kDa ultrafiltration tubes, and the two proteins were quantified by BCA method, and the protein concentration was finally adjusted to 1 mg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com