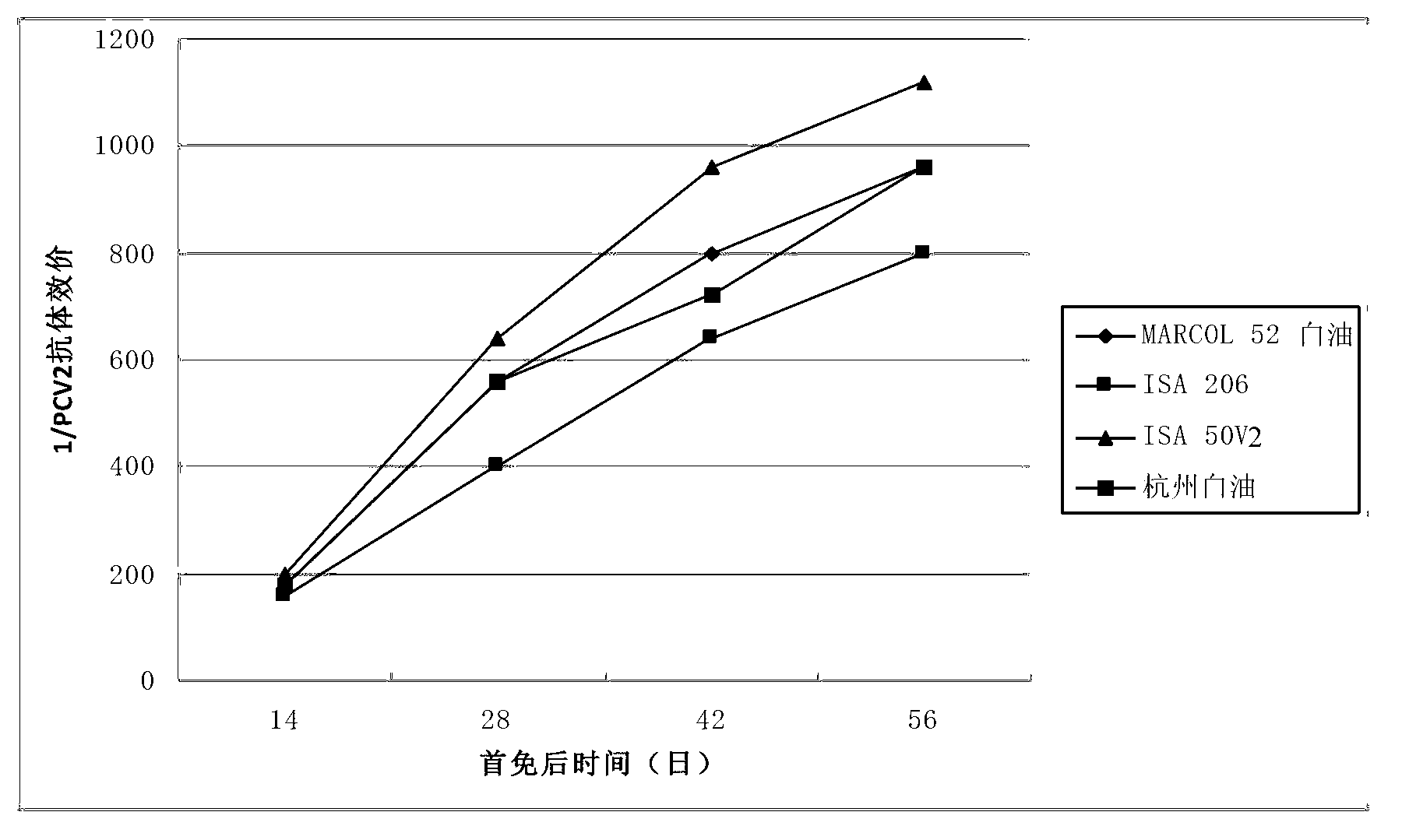
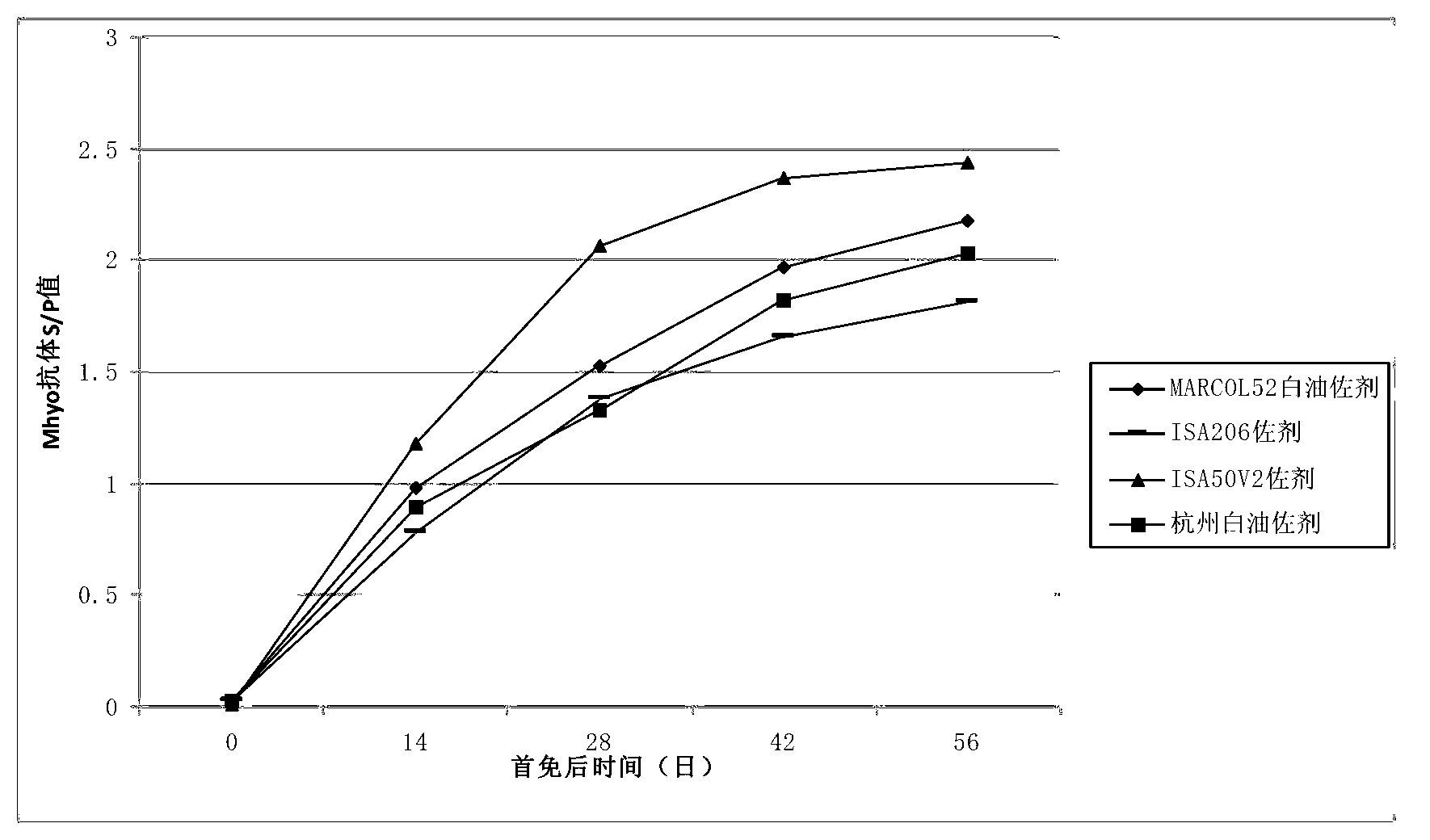
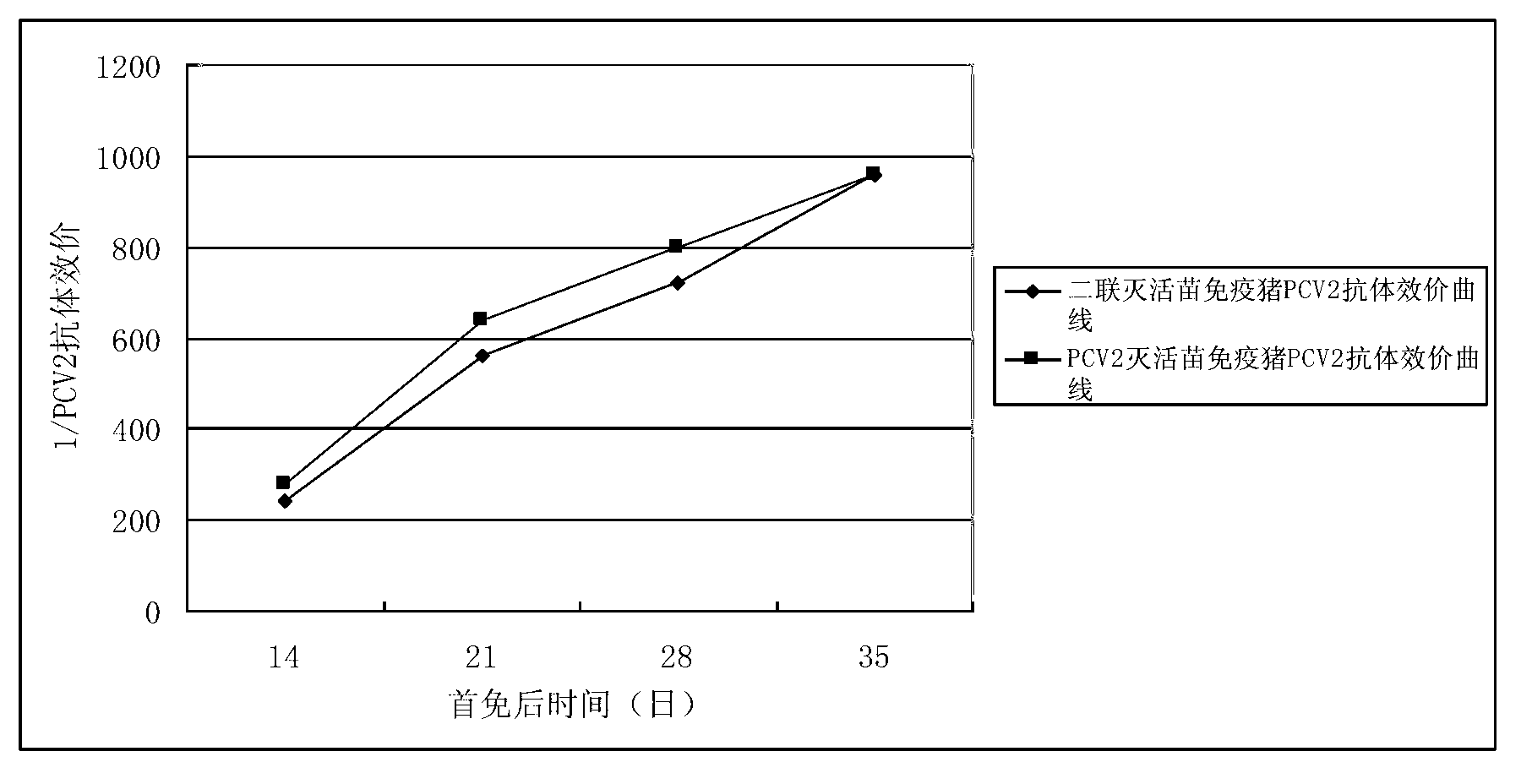
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
A dual inactivated vaccine and mycoplasma pneumonia technology, applied in virus/bacteriophage, biochemical equipment and methods, antiviral agents, etc., to reduce stress, avoid immune paralysis, and avoid immune failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 swine mycoplasma pneumonia inactivated vaccine
[0040] (1) Procedure for multiplying Mycoplasma hyopneumoniae
[0041] ① Propagation of Mycoplasma hyopneumoniae: inoculate Mycoplasma hyopneumoniae liquid medium (recipe see CN201110001231.0) with Mycoplasma hyopneumoniae at a ratio of 10% (V / V), culture at 37°C for 9 days, the color of the medium turns yellow and the pH drops. Harvest the bacterium liquid at 6.8 o'clock, and the amount of the harvested bacterium liquid is 50000ml. The live bacterial titer of Mycoplasma hyopneumoniae (DJ-166 strain) was 10 9 CCU / ml.
[0042] ② Concentration and purification of Mycoplasma hyopneumoniae: Concentrate the Mycoplasma hyopneumoniae liquid with an ultrafiltration membrane system to make the concentration of Mycoplasma hyopneumoniae liquid reach 10 11 ~10 12 CCU / ml, the amount of concentrated bacterial solution is 7600ml. Centrifuge the above bacterial solution at low speed and discard the prec...
Embodiment 2
[0057] Example 2 Protective test of different virulent challenge after immunizing pigs with swine mycoplasma pneumonia inactivated vaccine (DJ-166 strain)
[0058] In order to evaluate the immune effect of the inactivated Mycoplasma pneumoniae vaccine (DJ-166 strain), the pigs were immunized with the vaccine and challenged with strong virus strains from different sources, so as to investigate the protective effect of the vaccine against the strong virus prevailing in different places.
[0059] Use 15 Fujian black pigs 14-20 days old with negative serum antibody to Mycoplasma hyopneumoniae, inject 2.0ml of mycoplasma pneumoniae inactivated vaccine (DJ-166 strain) intramuscularly into each head, and set up 15 challenge control pigs at the same time, inject intramuscularly into each head Normal saline 2.0ml, isolated under the same conditions. Twenty-eight days after immunization, the immunization group and the challenge control group were randomly divided into 3 groups with 5 an...
Embodiment 3
[0065] Example 3 Comparative research test on the immune efficacy of porcine mycoplasma pneumonia inactivated vaccine and similar products
[0066] The inactivated vaccine for Mycoplasma pneumoniae in swine (strain DJ-166) prepared according to Example 1 was compared with the inactivated vaccine for Mycoplasma pneumoniae in swine (batch number A063229) produced by Pfizer in the United States in terms of immune efficacy and immune generation period.
[0067] Vaccine efficacy test: 15 Fujian black pigs 14-20 days old with negative serum antibodies to Mycoplasma hyopneumoniae were divided into 3 groups, each head of the first group was intramuscularly injected with 2.0ml of mycoplasma pneumonia inactivated vaccine (DJ-166 strain), and the first group Each head of the 2 groups was intramuscularly injected with 2.0ml of inactivated vaccine against mycoplasma pneumonia produced by Pfizer of the United States, and the third group was the challenge control group. Breeding in isolation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com