Swine mycoplasma pneumonia inactivated vaccine and preparation method thereof
A technology of Mycoplasma swine pneumonia and inactivated vaccines, which is applied in the direction of bacterial antigen components, oil/fat/wax non-effective ingredients, antibacterial drugs, etc., can solve the problems of complicated operation and difficult promotion, and achieve good spirits, strong immune effects, The effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 swine mycoplasma pneumonia inactivated vaccine
[0025] 1. Strain characteristics
[0026] 1.1 Morphological and biochemical properties
[0027] Mycoplasma hyopneumoniae P-5722-3 strain, the shape of the bacteria is ring-shaped, spherical, filamentous, rod-shaped or point-shaped polymorphic microorganisms, without cell walls, and Gram staining is negative.
[0028] 1.2 Culture characteristics
[0029] In the Lps liquid medium, cultured at 37°C for 5-10 days, the pH value of the medium dropped below 0.5 and appeared slightly turbid; on the solid medium, cultured at 37°C with 5% CO 2 Cultivate in a humid environment for 7 to 10 days, gray-white mycoplasma colonies can be seen under a low-power microscope. Most of the colonies have neat edges, a few colonies have irregular edges, and most of the colonies do not have "umbilical" features in the middle.
[0030] 1.3 Serological characteristics
[0031] It is identified by metabolic inhibi...
Embodiment 2
[0043] The safety test of embodiment 2 swine mycoplasma pneumonia inactivated vaccine
[0044] According to the preparation method of Example 1 of the present invention, 3 batches of inactivated vaccines for mycoplasma pneumonia were trial-produced, and the safety tests of non-target animals, single dose, overdose and single dose repeated injection were carried out respectively for the 3 batches of inactivated vaccines for trial-produced mycoplasma pneumonia .
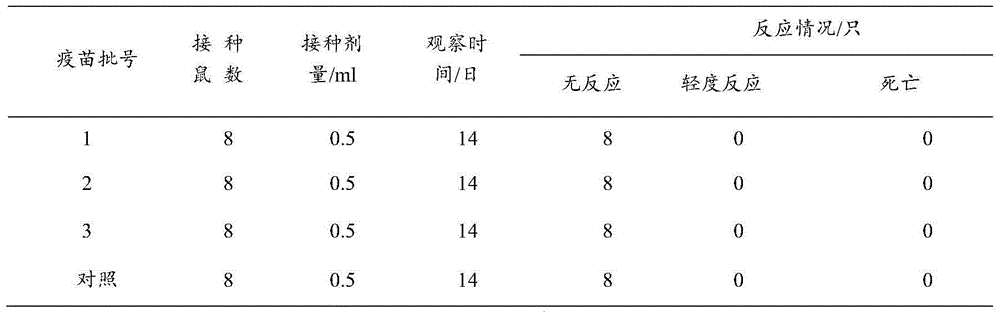
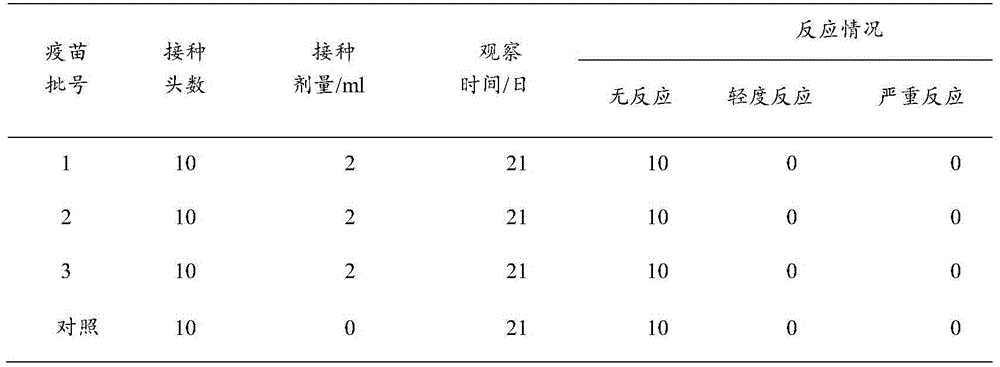
[0045] 1. Safety test of inactivated vaccine against Mycoplasma pneumoniae in non-target animals
[0046] For the safety test of mice, 3 batches of trial-produced vaccines were subcutaneously injected into 8 mice weighing 14-24g, each with 0.5ml. At the same time, a normal saline immunization control group was set up and observed for 14 days to observe whether there were any adverse reactions caused by the vaccine.
[0047] The results are shown in Table 1. The vaccinated mice did not have any adverse reactions, and a...
Embodiment 3
[0066] The immunity test of embodiment 3 swine mycoplasma pneumonia inactivated vaccine
[0067] According to the preparation method of Example 1 of the present invention, 3 batches of inactivated vaccines against Mycoplasma swine pneumonia were trial-produced.
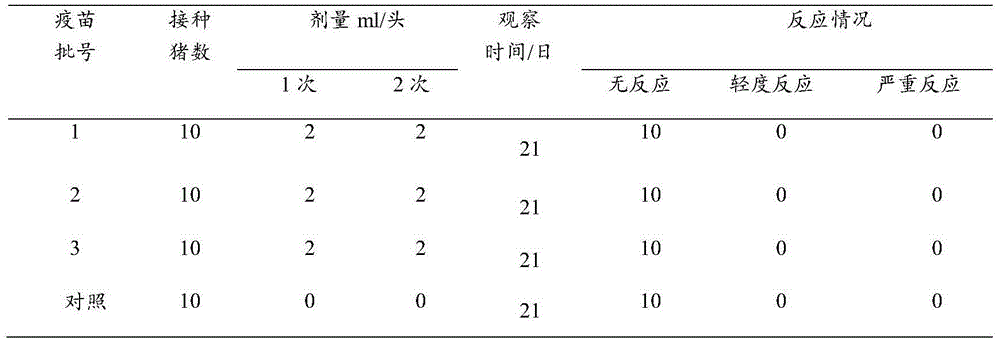
[0068] 1. Determination of the immune effect of inactivated vaccines
[0069] Three batches of trial-produced inactivated vaccines for Mycoplasma hyopneumoniae were used to intramuscularly inject 10 healthy susceptible pigs each with negative serum antibody against Mycoplasma hyopneumoniae, each with 2.0ml, and another 10 pigs were injected with normal saline as control pigs. 14 days after the injection, the same dosage was used for the second immunization. All experimental pigs were managed according to conventional feeding, and the piglets were weaned at the age of 24 days and transferred to the fattening house at the age of 65 days. After immunization, the birth weight, weaning weight, and slaughter weight of eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com