Immunoprotective antigen protein APJL_1976 for Actinobacillus pleuropneumoniae and application of immunoprotective antigen protein APJL_1976
A technology of protective antigen and actinobacillus, applied in the fields of application, vaccine, bacteria, etc., can solve the problems that limit the improvement and development of porcine pleuropneumonia subunit vaccine, and achieve the effect of strong immunogenicity and immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
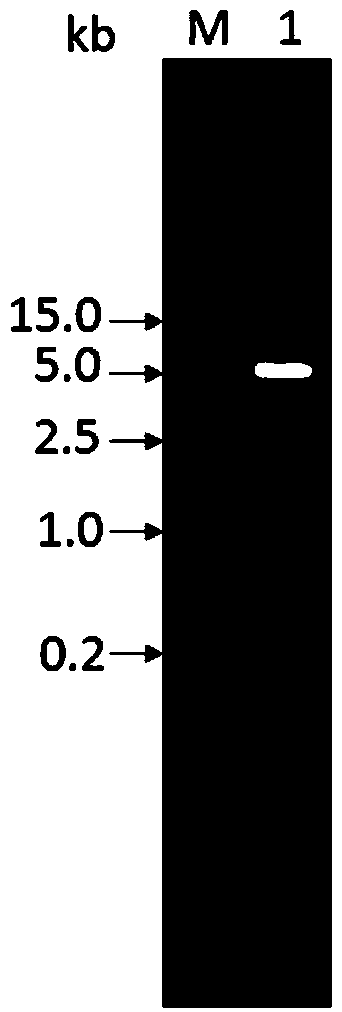
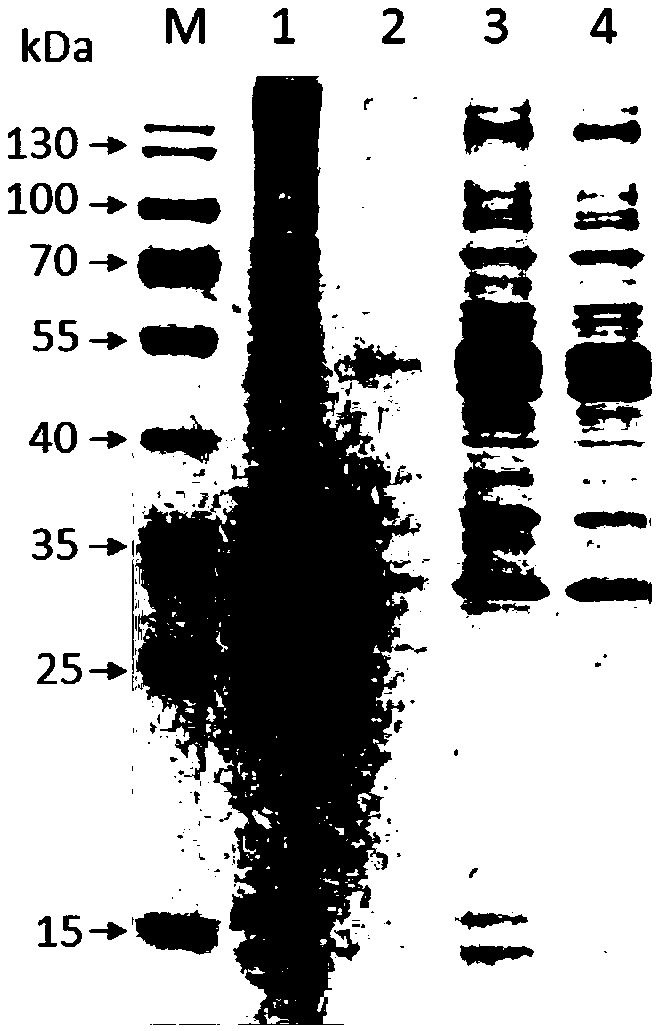
[0023] Expression of embodiment 1 APJL_1976 protein
[0024] 1. Genomic DNA extraction of Actinobacillus pleuropneumoniae
[0025] Take 1 mL of overnight cultured Actinobacillus pleuropneumoniae (JL03), centrifuge at 12,000 rpm for 1 minute, discard the supernatant, and then extract the genome of the JL03 strain according to the instructions of the genome extraction kit (Wuhan Boyue Biotechnology Co., Ltd.). The specific process is as follows: Add 200 μL RB to the bacterial pellet to resuspend, centrifuge at 10,000 rpm for 30 seconds, discard the supernatant, then add 200 μL RB to resuspend the pellet, then add 20 μL lysozyme to the centrifuge tube for vigorous shaking, and place at 37°C Incubate in the incubator for 15 minutes, then add 200 μL of binding solution CB, mix upside down, add 20 μL proteinase K (20 mg / mL), mix well, put it in a 70°C water bath for 10 minutes, take it out and add 100 μL iso Propanol, mix well by inversion, then transfer all the substances in the c...
Embodiment 2
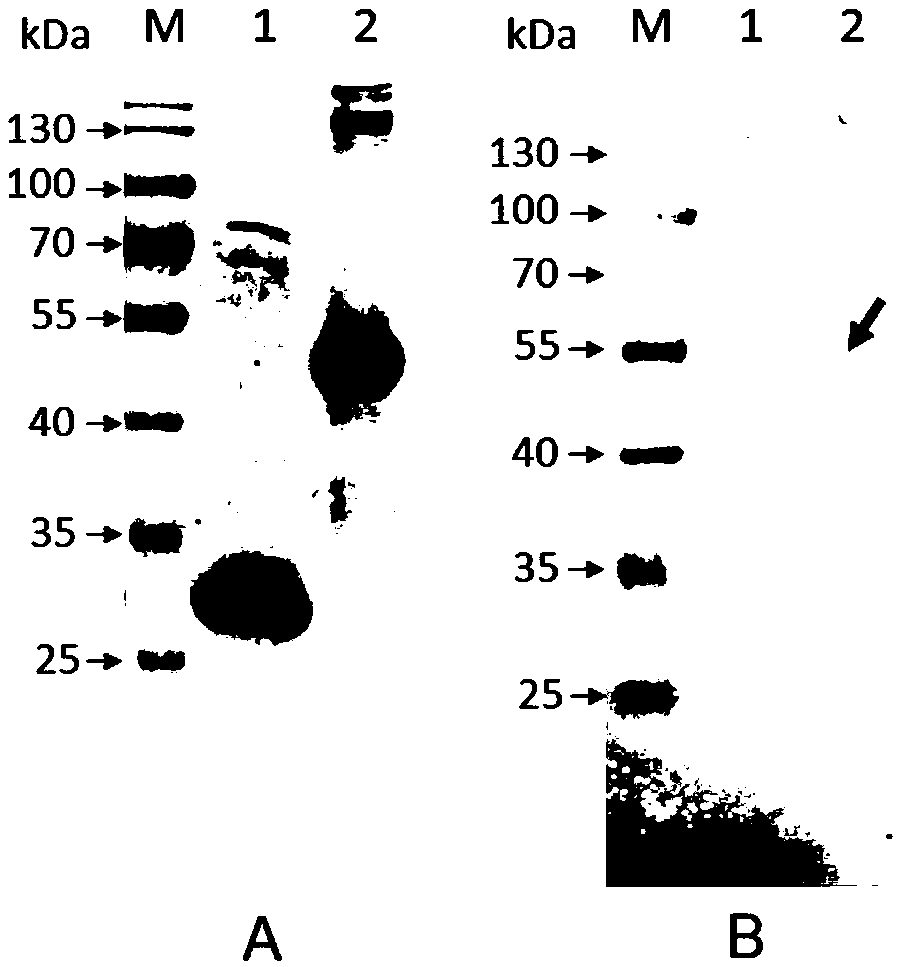
[0056] Example 2 Identification of the immune protection of APJL_1976 protein
[0057] (1) Active immunization and challenge of mice
[0058] Six-week-old female BALB / c mice (purchased from the Experimental Animal Center of Hubei Provincial Center for Disease Control and Prevention) were immunized with APJL_1976 protein and GST protein purified in Example 1, 12 in each group. At the same time, a TSB blank control was set without immunization. The first immunization dose was 80 μg / mouse, mixed with an equal volume of Freund's complete adjuvant, emulsified, and injected subcutaneously at multiple points on the back of the mouse. After an interval of two weeks, the second immunization was carried out, the dose was 80 μg / mouse, mixed with an equal volume of Freund’s incomplete adjuvant, emulsified, and injected subcutaneously at multiple points on the back of the mouse. After 10 days, the antibody level was detected by ELISA, and HRP Labeled goat anti-mouse IgG was used as the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com