Porcine mycoplasmal pneumonia and porcine infectious actinobacillus pleuropneumoniae serum 1 type gene engineering strain vaccine and application thereof
A genetically engineered vaccine, the technology of mycoplasma pneumonia, is applied in the field of livestock infectious diseases, which can solve the problems of pig stress and increased breeding costs, and achieve the effects of clear induction sites, high biological safety, and stimulation of mucosal immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 constructs intermediate transfer plasmid
[0045] 1. Design of primers (for gene cloning and molecular detection shown in Table 1)
[0046] Table 1: PCR Primers
[0047]
[0048]
[0049] The underlined parts in the primer sequences in the table are the corresponding restriction sites. The underlined parts after the primer sequences designed for the P36 gene are restriction sites and ribosome binding sites respectively. The primers in Table 1 were provided by Shanghai Sangon Bioengineering Technical Service Company Synthesis.
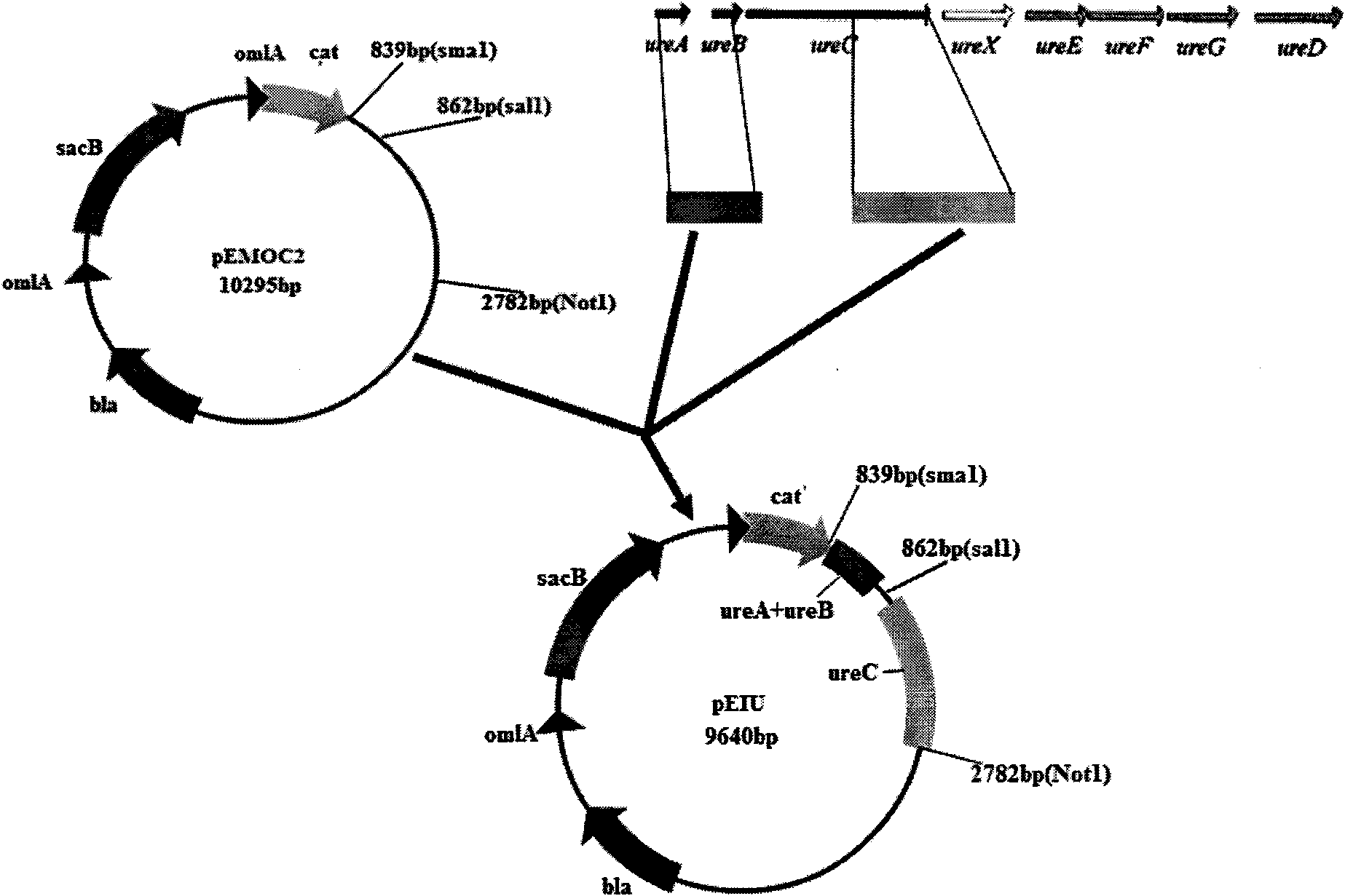
[0050] 2. Amplification of urease gene homology arm, strong promoter sequence and P36 gene
[0051] Using the genome of APP serotype 1 attenuated bacteria (SLW03) as a template, primer pairs P5 and P6, P7 and P8 were used to amplify the upper and lower homology arms respectively. The PCR amplification reaction system is: 10×Taq Buffer5.0μl (including mg 2+ ), 2.5mmol / L dNTPs 4μl, 20μmol / L upstream and downstream primers 1.0...
Embodiment 2
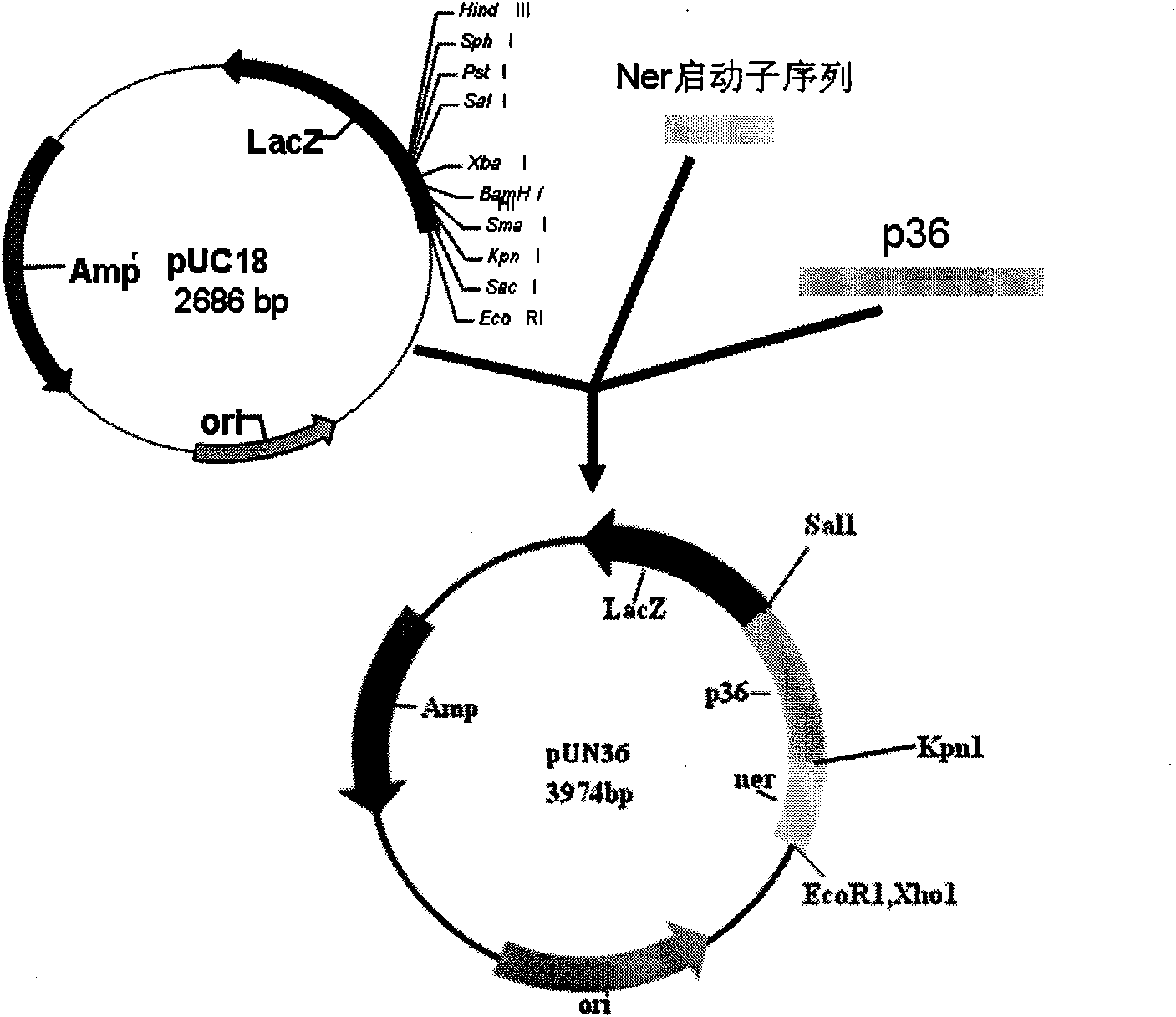
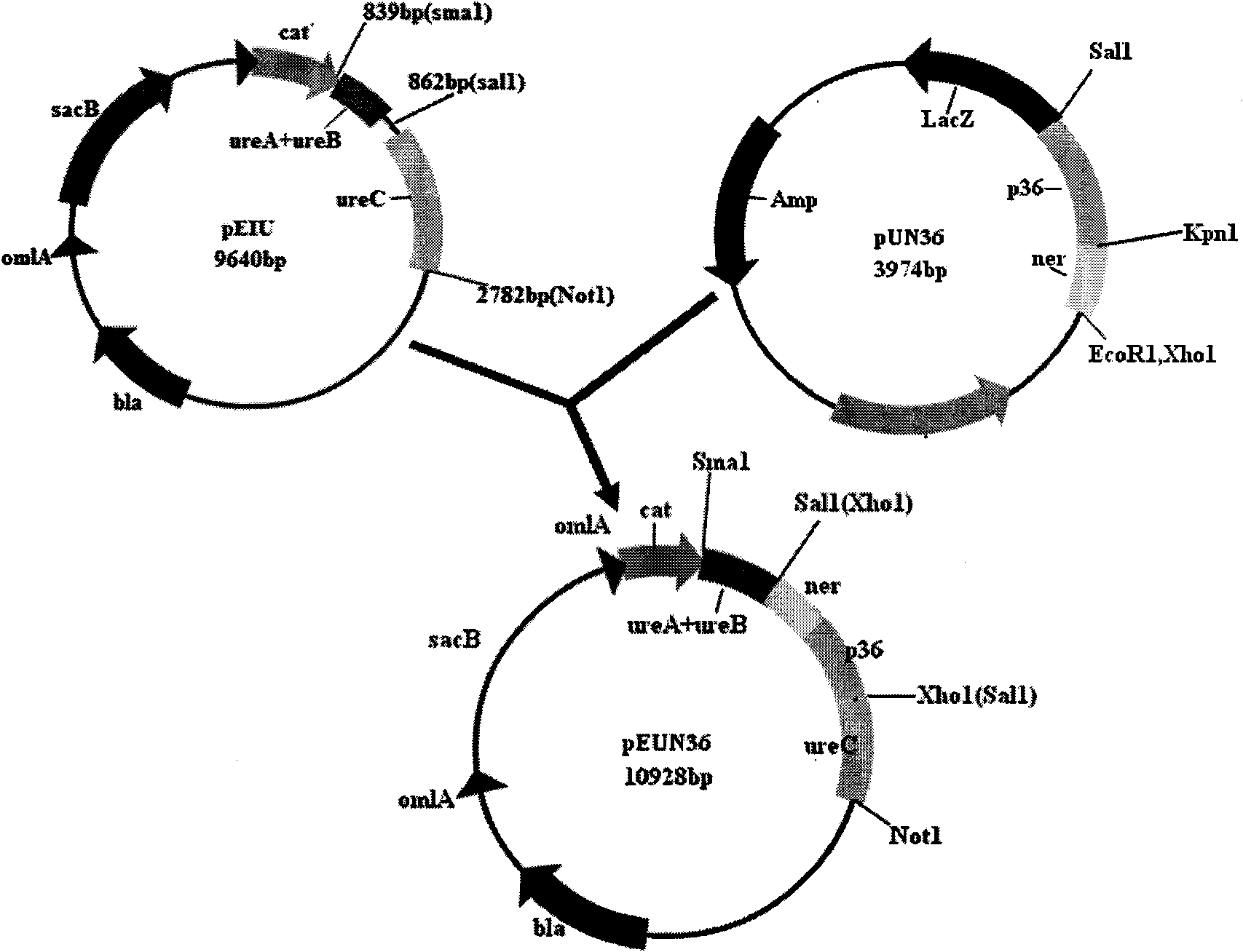
[0054] Example 2 Construction of intermediate transfer plasmids pEIU, pUCN36 and pEUN36
[0055] After the PCR product was recovered and purified, the homologous right arm and the pEMOC2 vector were digested with Sal I and Not I, recovered and purified, ligated, transformed into Escherichia coli DH5α, positive clones were screened, a small amount of plasmid was extracted, and restriction endonuclease was used to The recombinant plasmid was identified by enzyme digestion, and its size was confirmed to be in line with the expectation by 0.8% agarose gel electrophoresis; then the homologous left arm and pEMOC2 containing the homologous right arm were double-digested with Sma I and Sal I, recovered and purified Ligation, transformation of Escherichia coli DH5α, screening of positive clones, small extraction of plasmids, identification of recombinant plasmids with restriction endonucleases, and 0.8% agarose gel electrophoresis, confirmed that the size was in line with expectations; ...
Embodiment 3
[0058] Example 3 conjunctive transfer
[0059] The constructed recombinant plasmid pEUN36 was transformed into Escherichia coli X7213 by the heat shock method (translated by Huang Peitang et al. Sambrook J, Russell D W, Molecular Cloning Experiment Guide (Third Edition), Beijing: Science Press, 2002), and obtained The positive Escherichia coli and Actinobacillus pleuropneumoniae type 1 attenuated strain SLW03 parental strain were cultured overnight, the collected bacteria were washed twice with sterile PBS, and the bacterial concentration was adjusted to OD 600 is 0.8. Take 100 μl of bacterial suspension and mix them, paste the sterile nitrocellulose filter membrane on the TSA solid plate containing NAD and DAP, drop the mixed bacteria solution on the filter membrane, cultivate overnight at 37°C, and make donor and acceptor controls at the same time . Wash the bacterial solution on the filter membrane, wash it twice with sterile PBS, and coat the TSA plate containing TSA and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com