Vaccine of swine fever-porcine contagious pleuropneumoniae bivalent subunit and preparation method and applications thereof
A technology of porcine pleuropneumonia and pleuropneumonia, which is applied in the field of vaccine and its preparation of two subunits of swine fever-porcine infectious pleuropneumonia, and can solve the problem of high cost of immunization alone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis, PCR amplification and identification of embodiment 1 classical swine fever virus E2 gene
[0079]Downloaded the published E2 sequence of classical swine fever virus from GenBank and the E2 sequence of new strains popular in recent years as a reference, carried out codon optimization and modification on the basis of the E2 gene of the C strain, and added a signal peptide sequence (VLRGQIVQGVIWLLLVTGAQG , SEQ ID NO.11), the 3' end is connected with a His tag composed of 6 histidines, and the modified sequence is synthesized by a gene synthesis company; after obtaining the synthesized E2 gene through PCR amplification, the amplified The product E2 gene was sequenced, and the measured E2 gene was compared with the E2 gene of the C strain and the reference strains of each subgroup. The results showed that the nucleotide homology between the finally obtained E2 gene and the E2 gene of the C strain Reaching 93.2%, as the target gene for constructing CSFV E2 recombin...
Embodiment 2
[0085] Example 2 Construction and identification of recombinant baculovirus of classical swine fever virus E2 protein
[0086] The construction method of the recombinant baculovirus of classical swine fever virus E2 protein is shown as a flow chart figure 2 shown. Using the synthetic swine fever virus E2 gene as a template, high-fidelity Pfu DNA polymerase was used for PCR reaction to amplify the swine fever virus E2 gene. After sequencing and verification, the recombinant virus was constructed by referring to the Invitrogen operation manual. Gene amplification and identification, construction of transfer vector, screening and identification of transfer vector, transfection of transfer vector and linear baculovirus, screening of recombinant virus, identification of recombinant virus and expression of E2 protein, and finally obtain a virulence strain The stable recombinant baculovirus with high E2 protein secretion was named CSFV-Rb-E2.
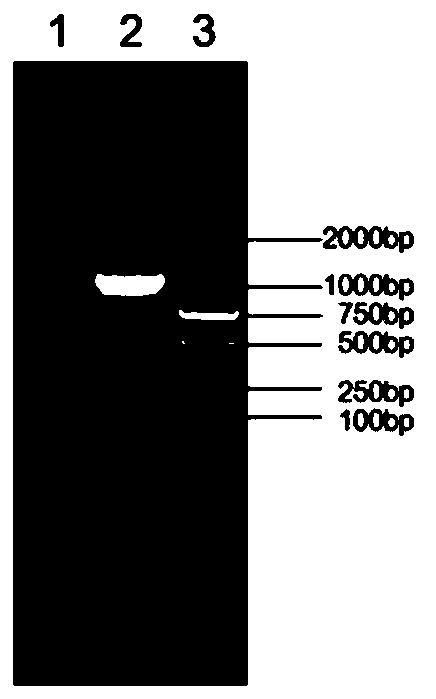
[0087] 1) Amplification of E2 gene ...
Embodiment 3
[0103] Expression condition optimization and harvest of embodiment 3 classical swine fever virus E2 protein
[0104] Using a 3×3 orthogonal experiment design, the three factors of inoculation dose, cell density at inoculation, and harvest time were compared and analyzed, among which three levels of inoculation doses were set at 0.1, 0.5, and 1 MOI, and 1 million, 2 million, and 3 million / There are three levels of cell density in ml, and the three conditions of collection time are 72, 96, and 120 hours. The E2 protein content in the supernatant harvested from the above experiments is detected by the double-antibody sandwich ELISA method. Finally, it was determined that when the Sf-9 cell density was 2 million / ml, it was inoculated with 1 MOI, and the expressed recombinant E2 protein had the highest content. It was verified by Western-blot that the protein had good immunogenicity. SDS-PAGE protein electrophoresis and gelation The gel imaging system comes with band analysis soft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com