Preparation method and applications of actinobacillus pleuropneumoniae-derived I-F type CRISPR-associated protein Csy4
A technology of porcine pleuropneumoniae and actinobacillus, applied in the field of gene editing and/or genome modification of organisms, and genetic engineering, can solve the problems of great differences in sequence homology among family members
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Construction of the I-F CRISPR-related protein Csy4 expression vector of Actinobacillus pleuropneumoniae
[0046] The pET28b vector was double digested with NcoI and XhoI. Both ends of the artificially amplified Csy4 gene have primers F-Csy4:GGTTA CCCATGG CCAGTGAATTAACACATTACAT and R-Csy4:GGTTAC CTCGAG GAAATGTGGGACGGTTGCT. , and then use T4 ligase to connect the double-digested Csy4 gene PCR product with the linearized pET28b vector to obtain the pET28b-Csy4 recombinant plasmid.
Embodiment 2
[0047] Embodiment 2: Expression of pET28b-Csy4 recombinant plasmid in Escherichia coli
[0048]The pET28b-Csy4 recombinant plasmid was transformed into BL21(DE3) Escherichia coli, and the LB solid plate was coated. After overnight culture, pick a single colony in 100ml LB liquid medium, overnight at 37°C and 250rpm, use it as seeds for later use, and inoculate the seeds in shake flasks containing 2L LB medium for cultivation the next day until OD 600 0.6, add IPTG, the final concentration is 0.4mM, and induce for 14h at 20°C and 180rpm;
[0049] Wherein, 50ug / L kanamycin was added to both the LB solid plate and the LB medium;
[0050] Each 1 LLB medium component includes: 5g yeast powder, 10g peptone and 10g sodium chloride;
Embodiment 3
[0051] Embodiment 3: Purification of Csy4 protein
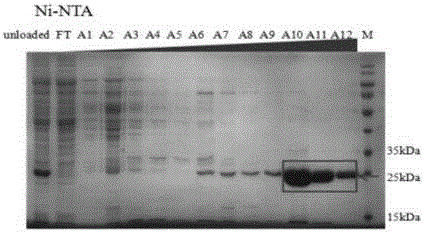
[0052] The cells after induction of expression were centrifuged at 5000rpm for 25min, discarded the supernatant, and resuspended with the lysate. The resuspended bacteria were crushed with a high-pressure homogenizer, centrifuged at 24,000 rpm for 1 h, and the supernatant was collected, purified with a Ni column, and then dialyzed to remove imidazole.
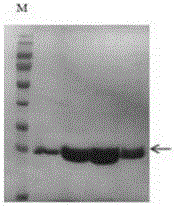
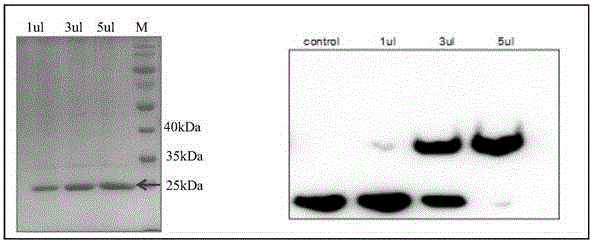
[0053] Such as figure 2 , image 3 As shown, after dialysis, the Csy4 protein was purified by molecular sieves to obtain a pure product, and dialysis reduced the salt concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com