Patents
Literature
125 results about "Inverted Terminal Repeat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inverted terminal repeats. [in¦vərd·əd ¦tər·mə·nəl ri′pēts] (cell and molecular biology) Related or identical sequences of deoxyribonucleic acid in inverted form occurring at opposite ends of some transposons.
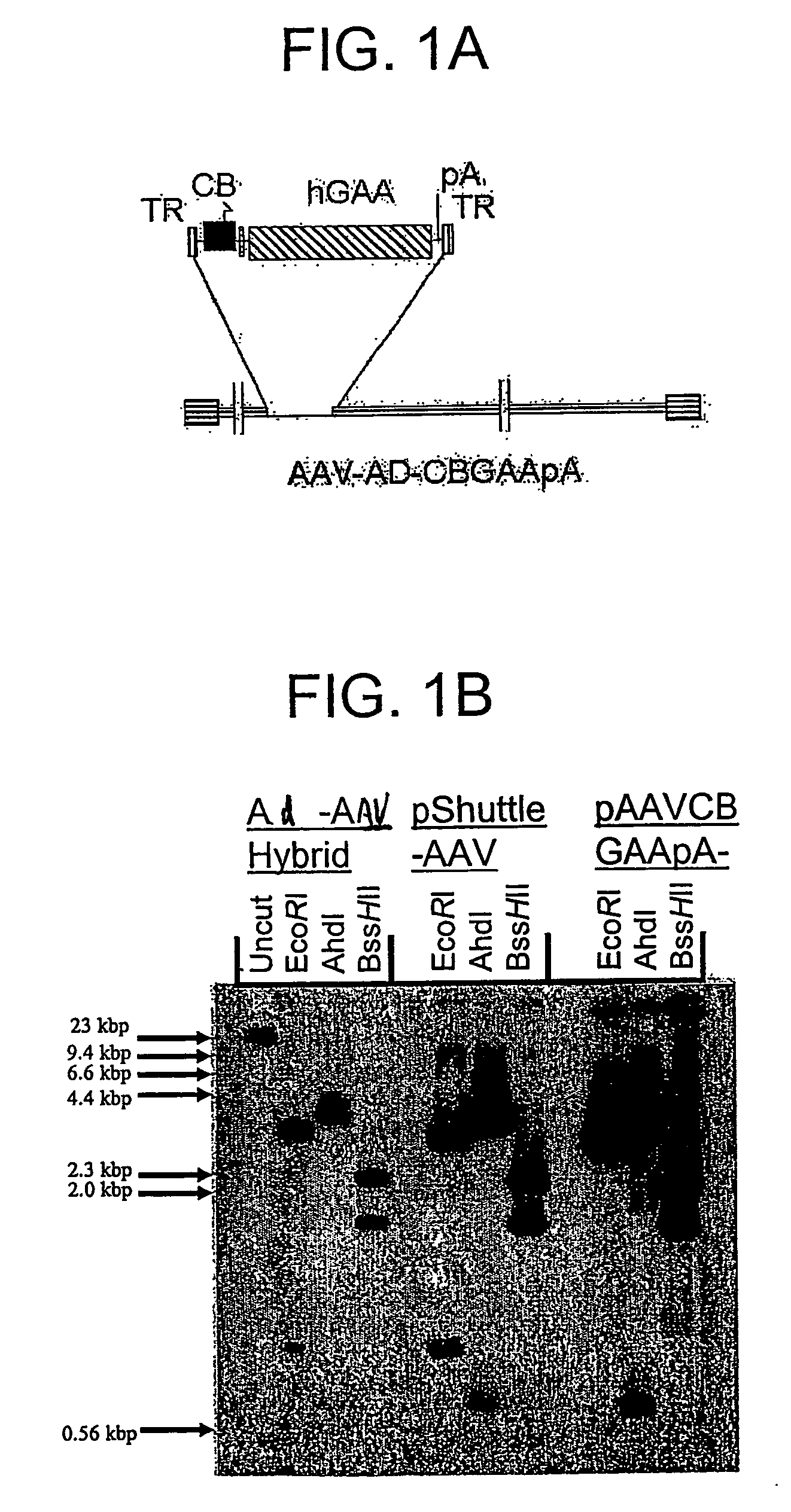
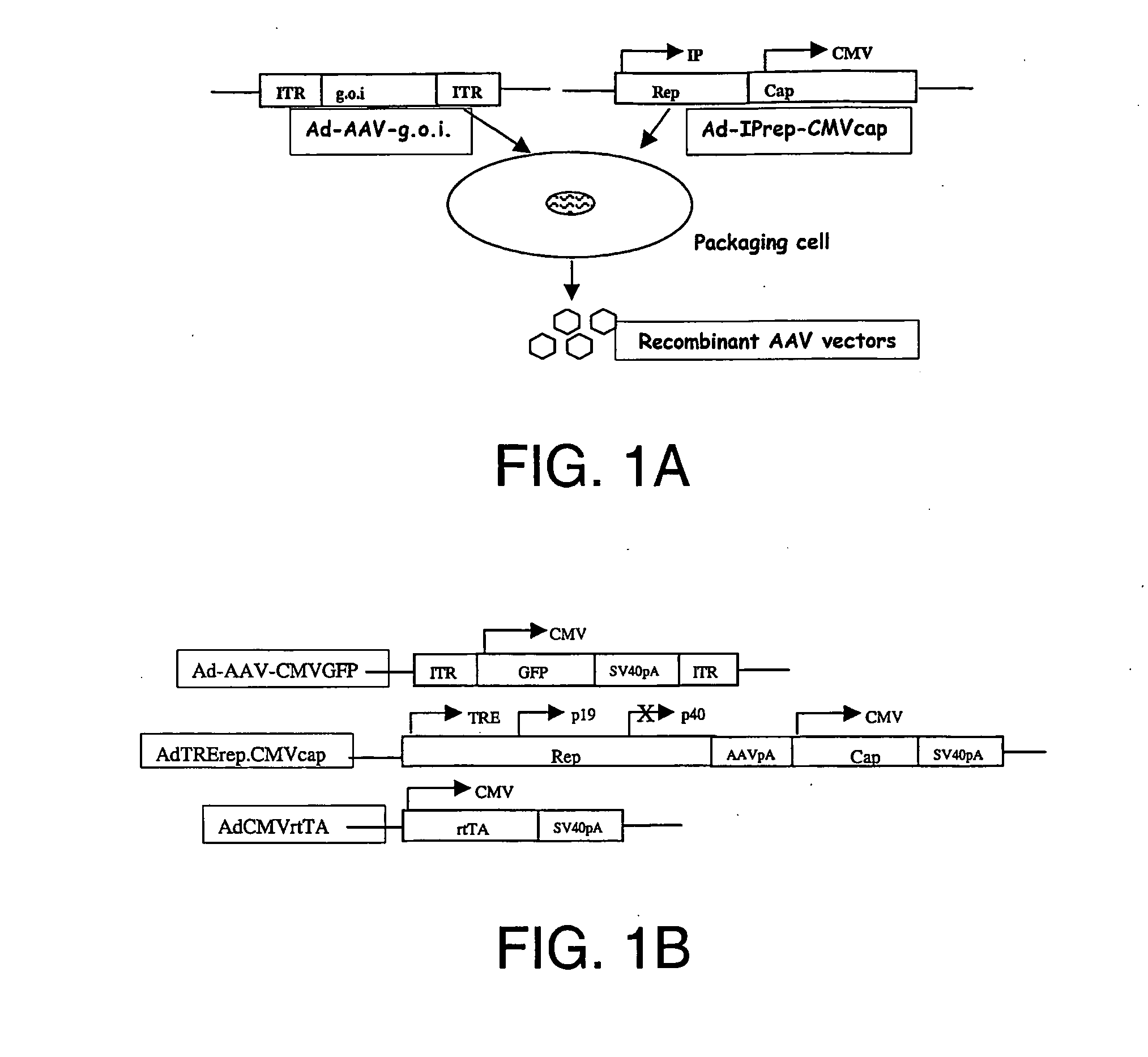
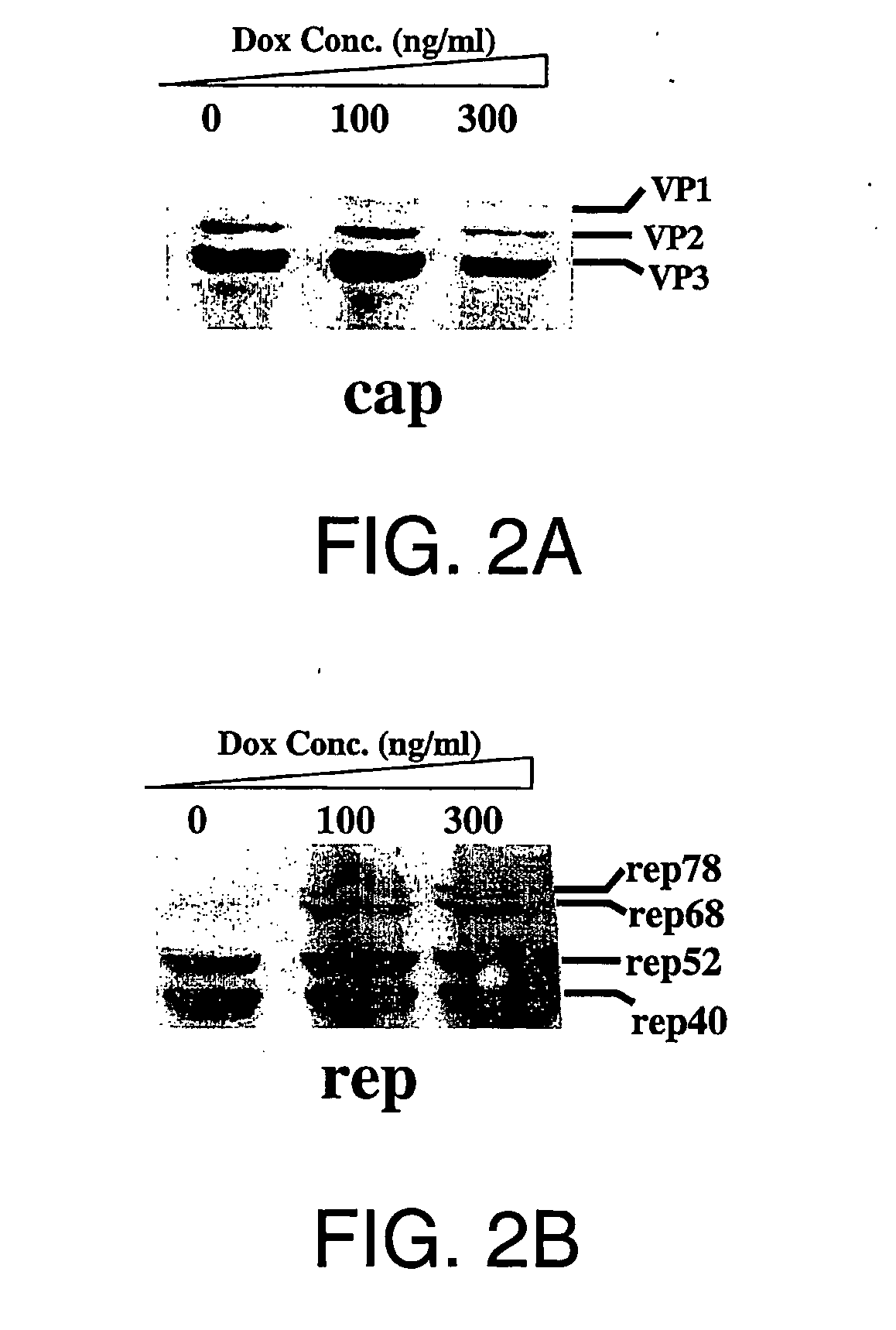
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
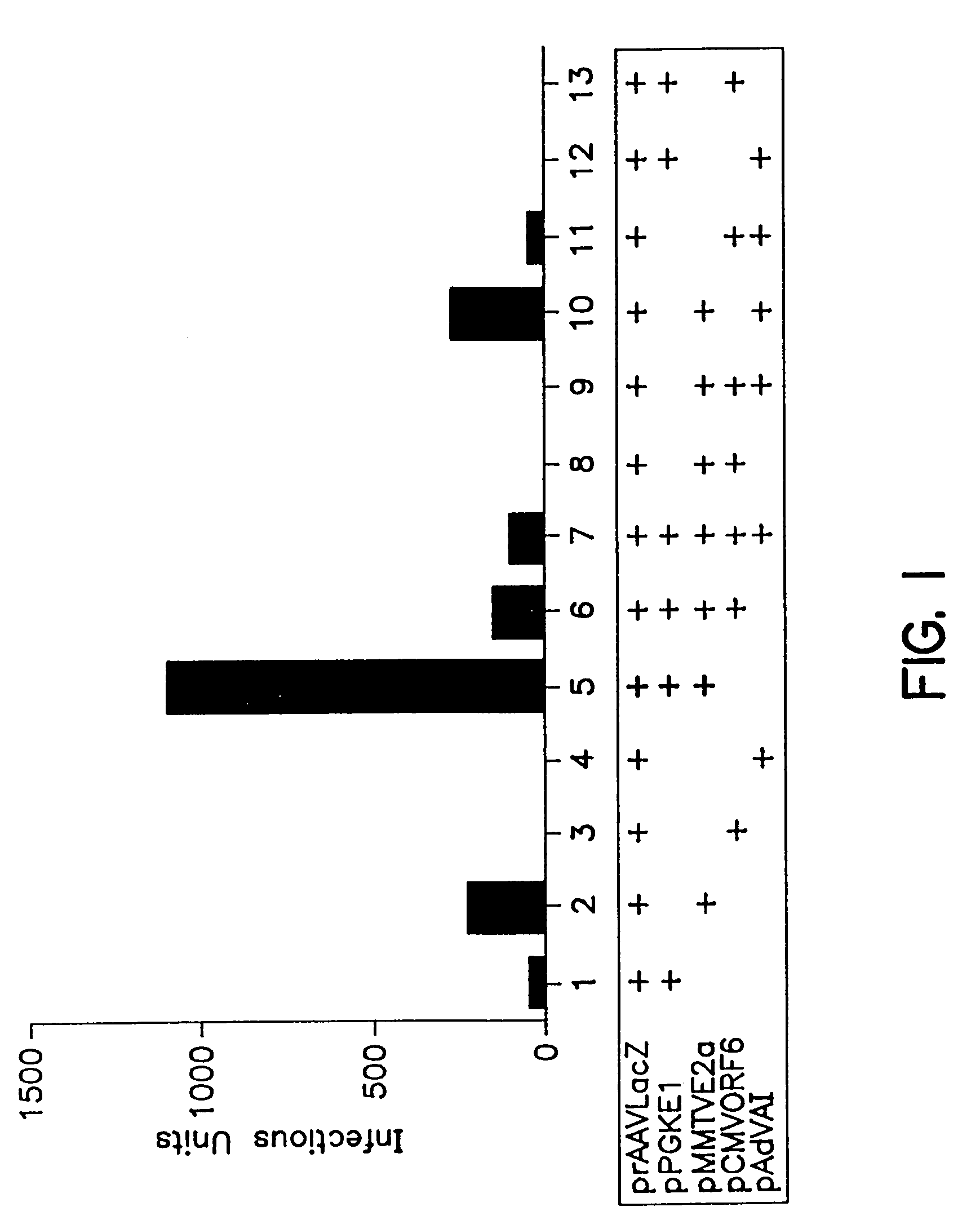
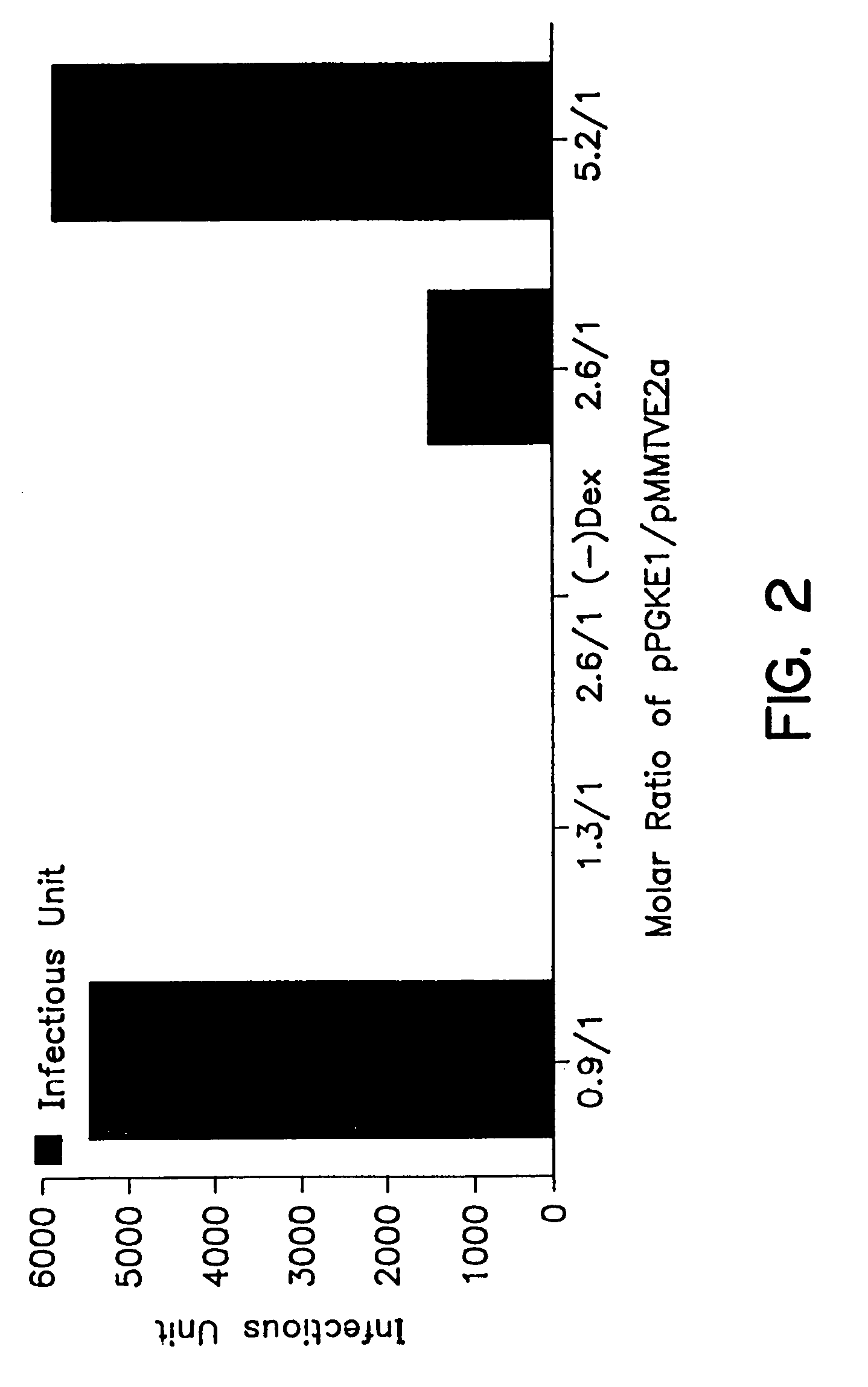
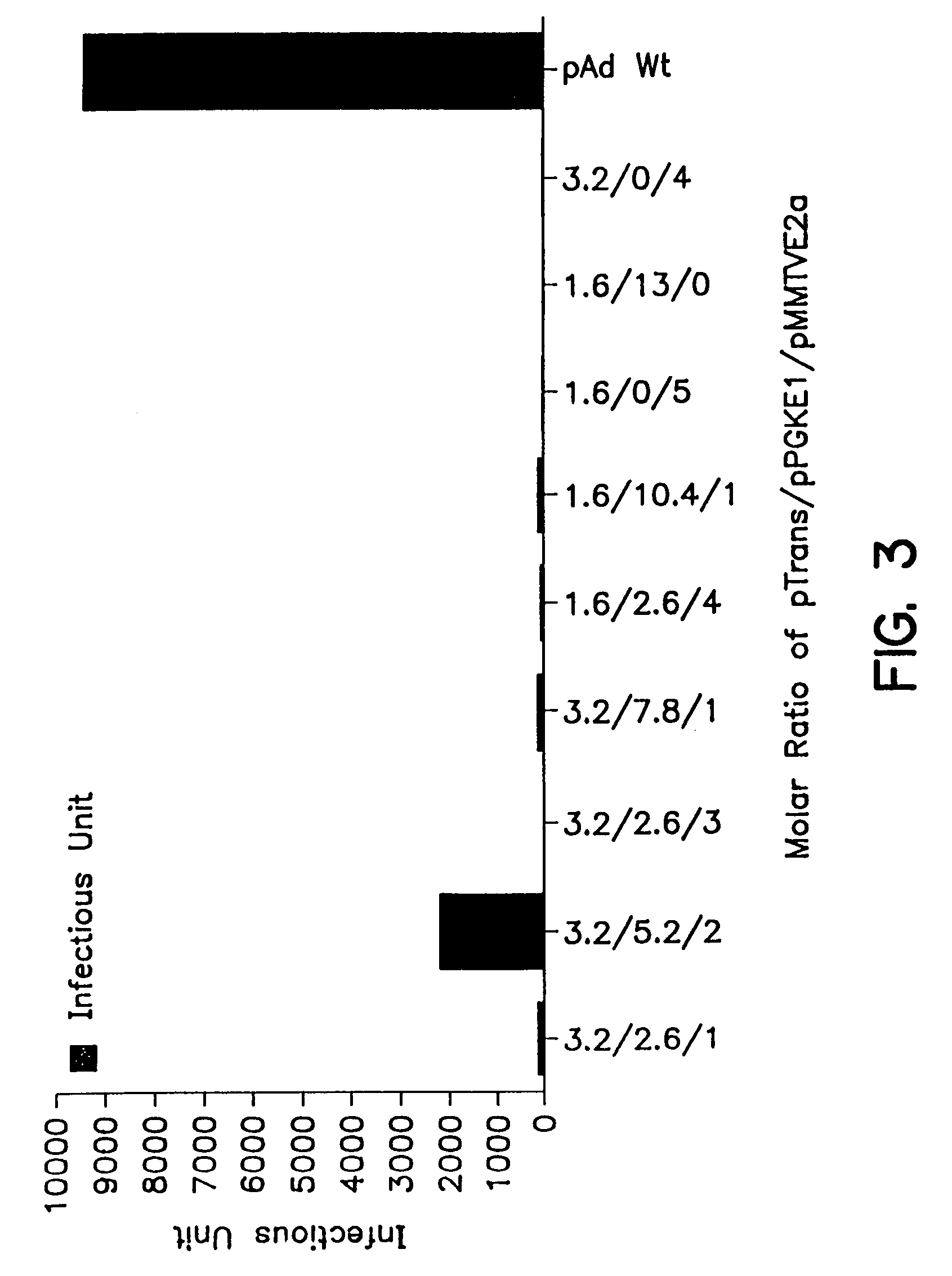
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
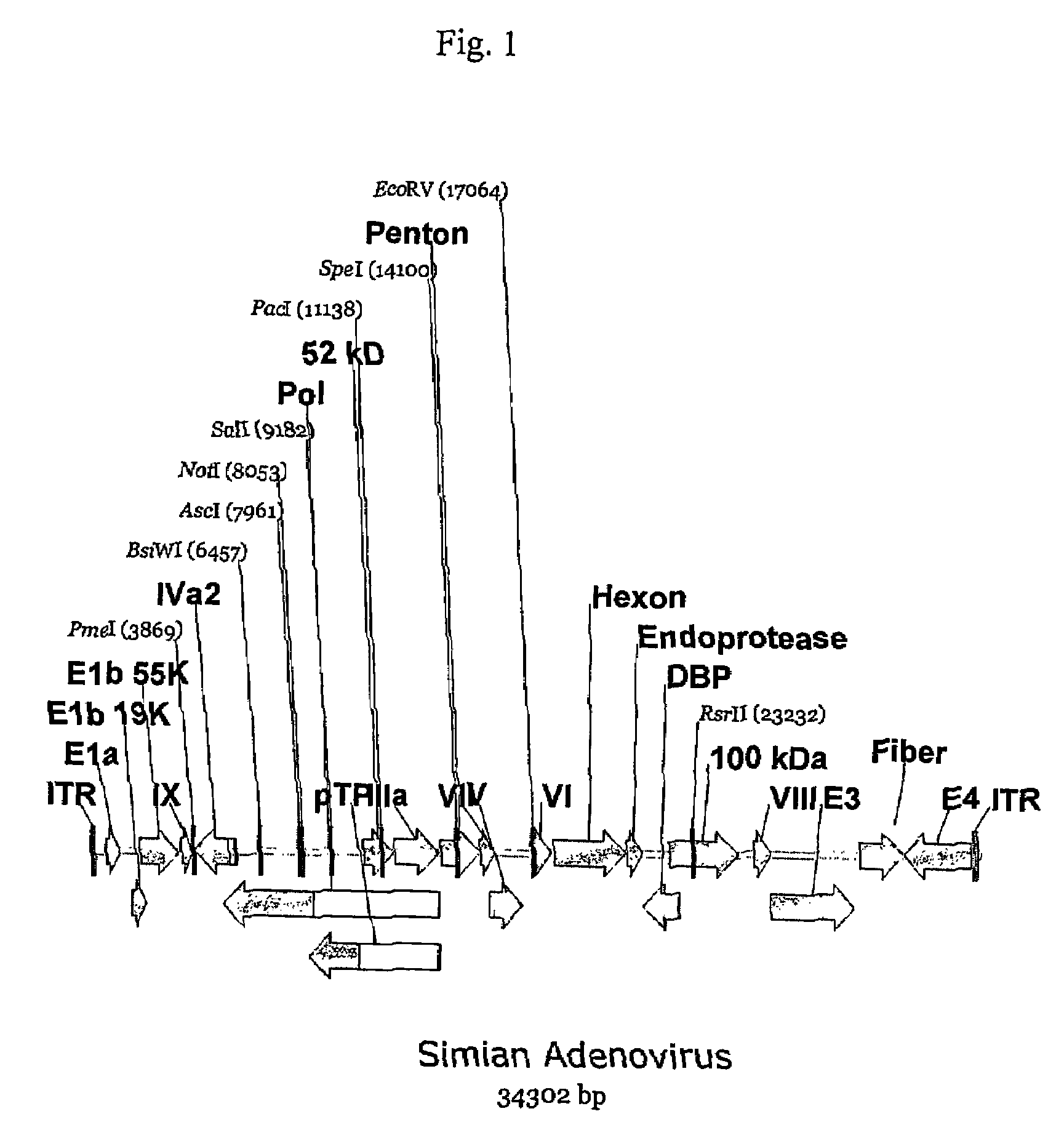
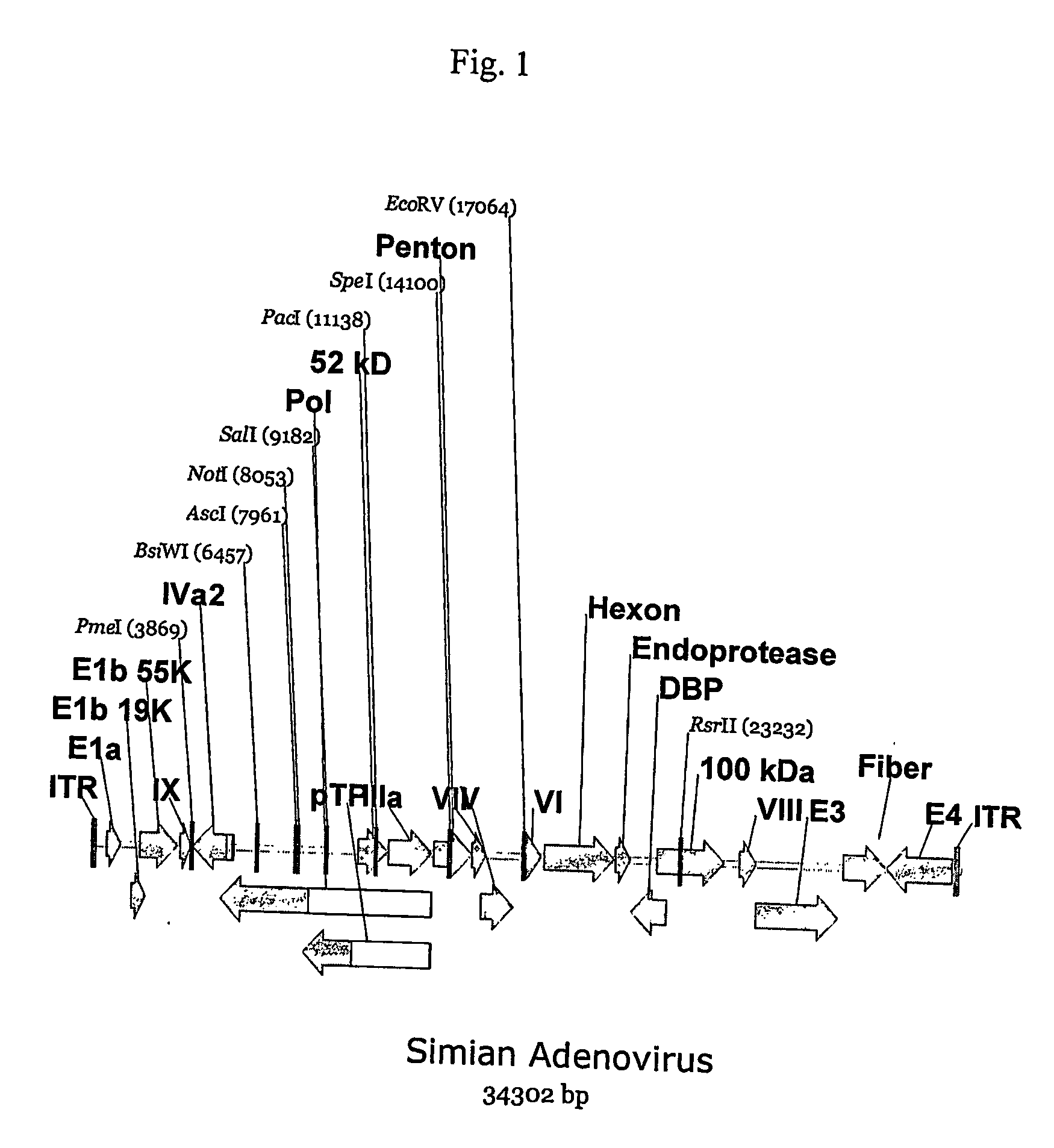
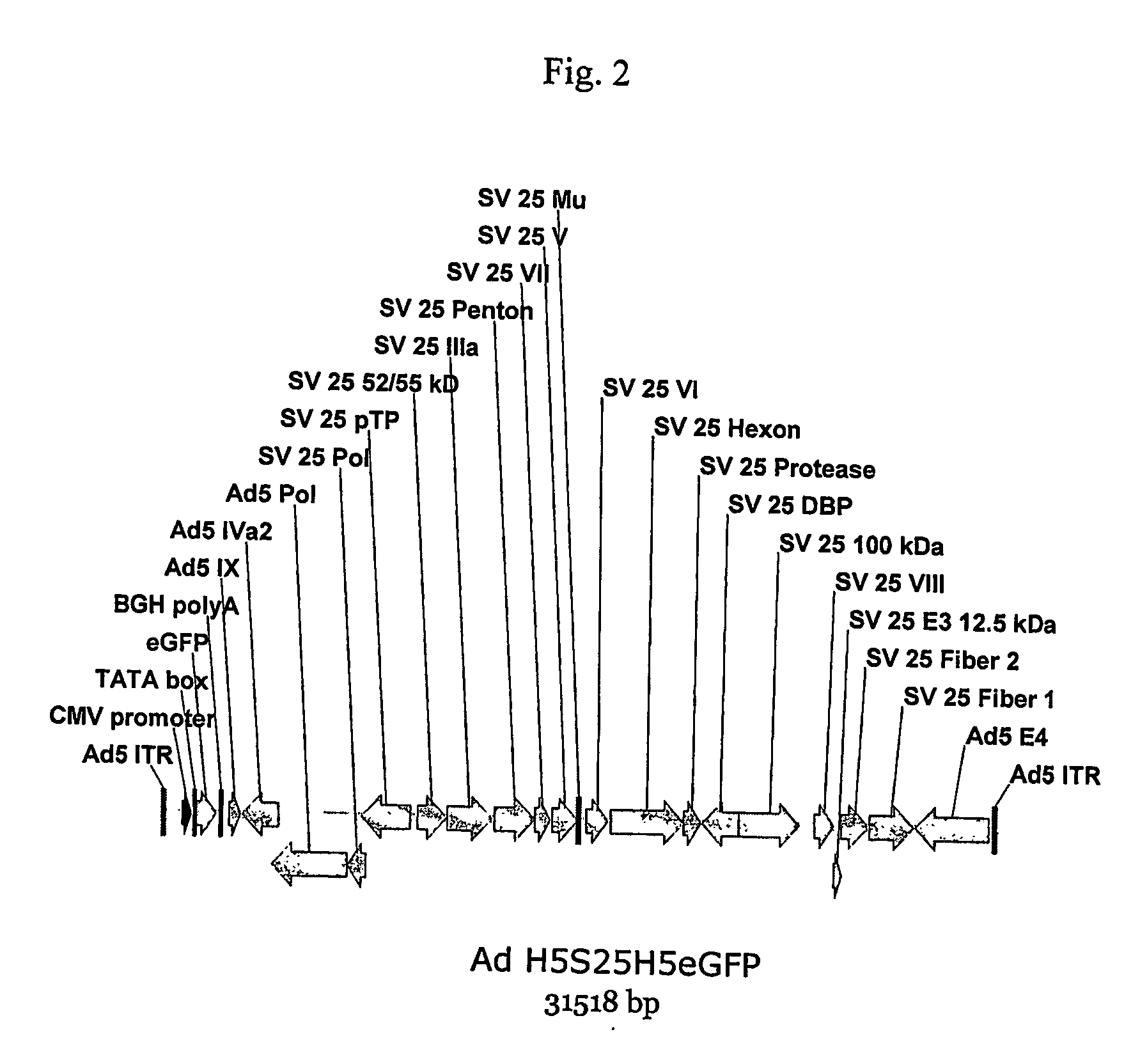
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
AAV vectors produced in insect cells
ActiveUS8163543B2Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
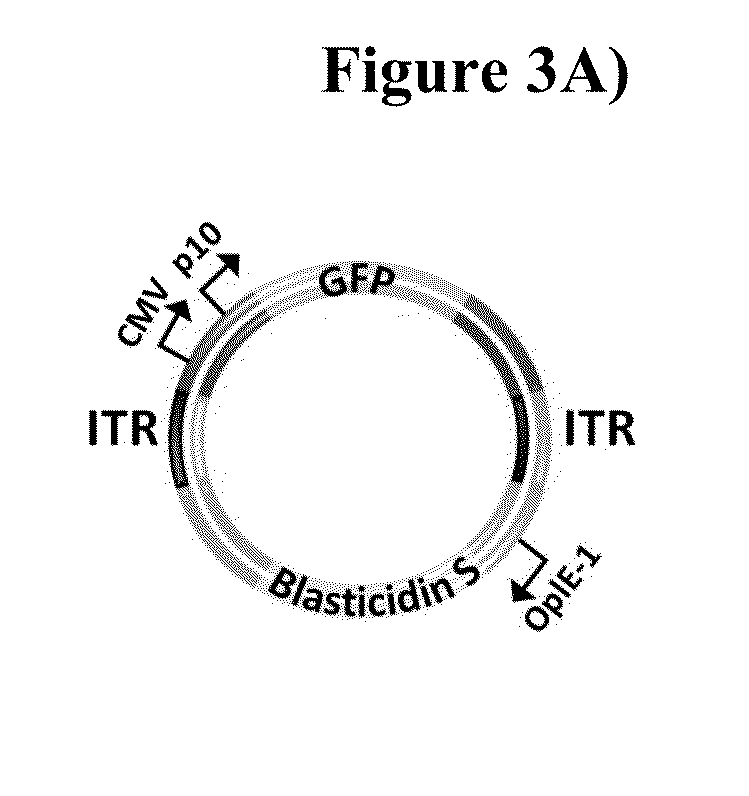
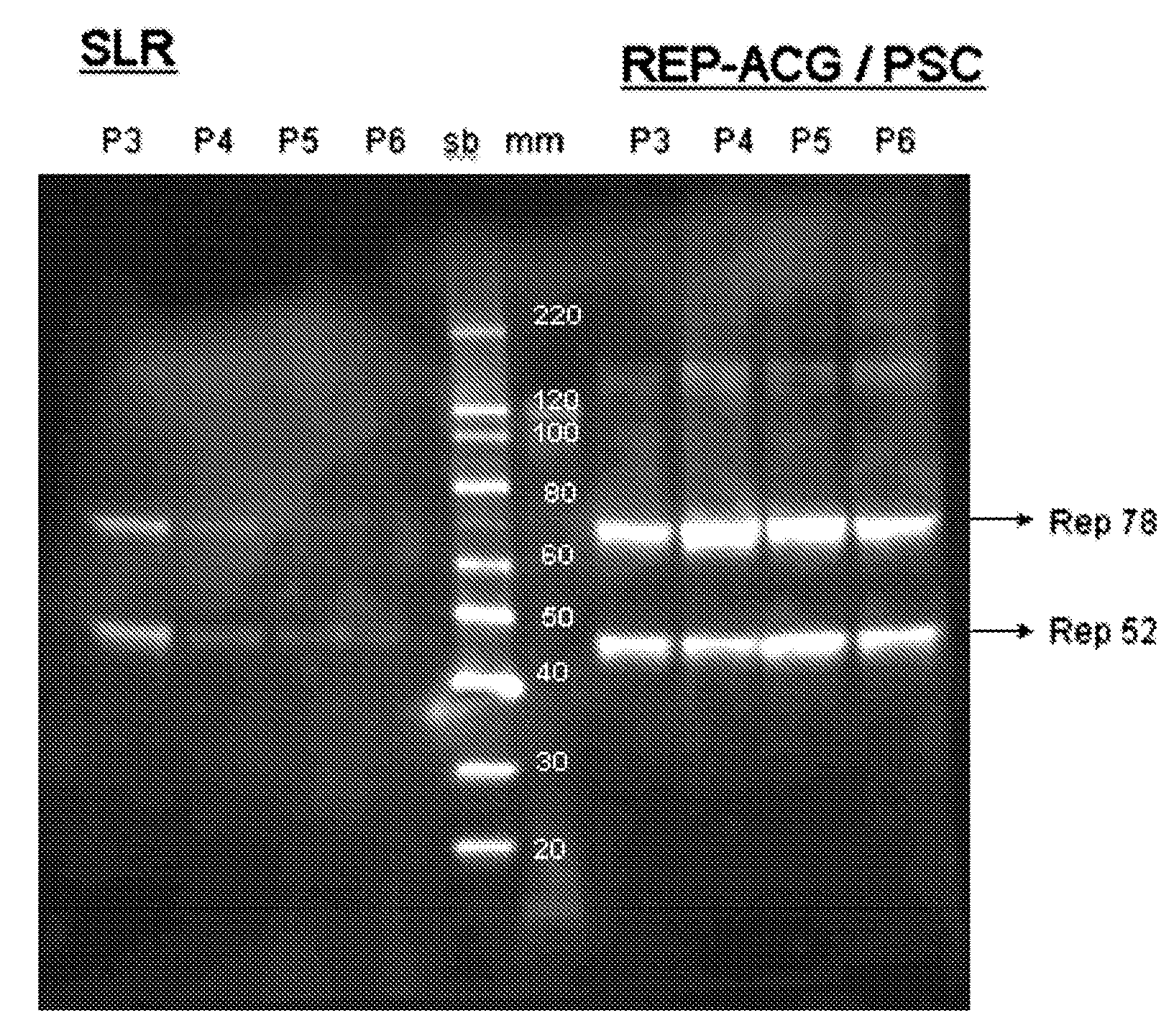
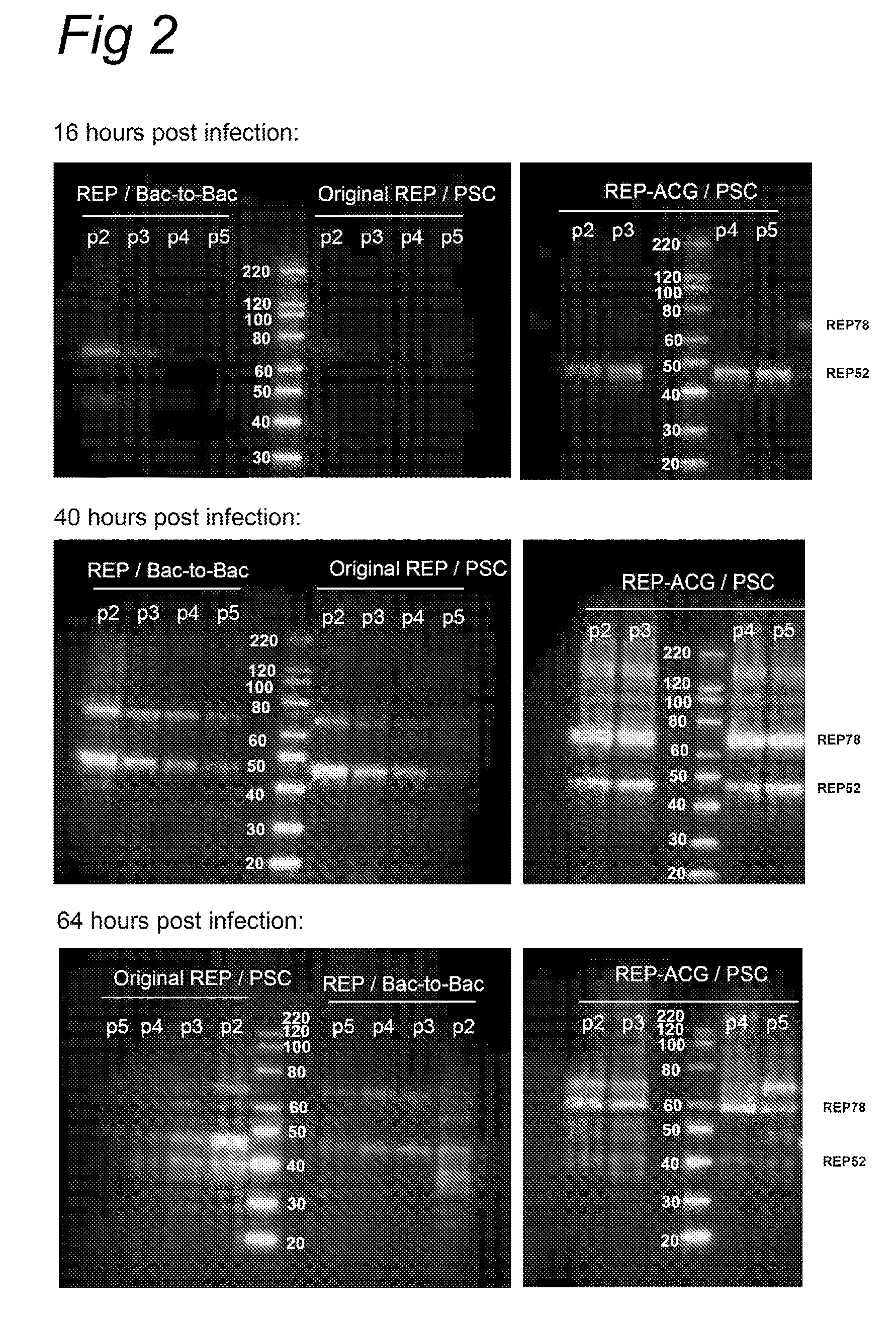
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
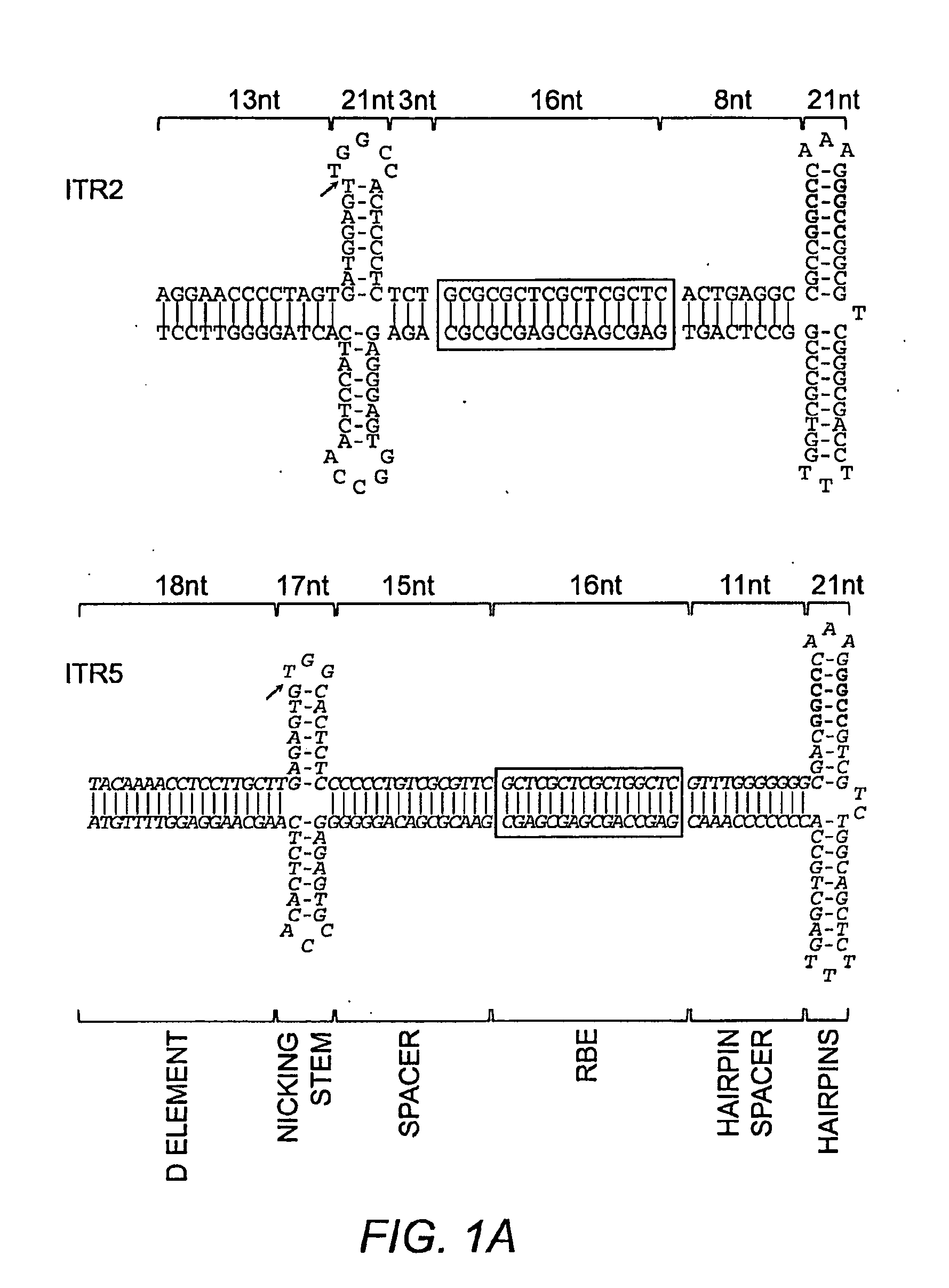
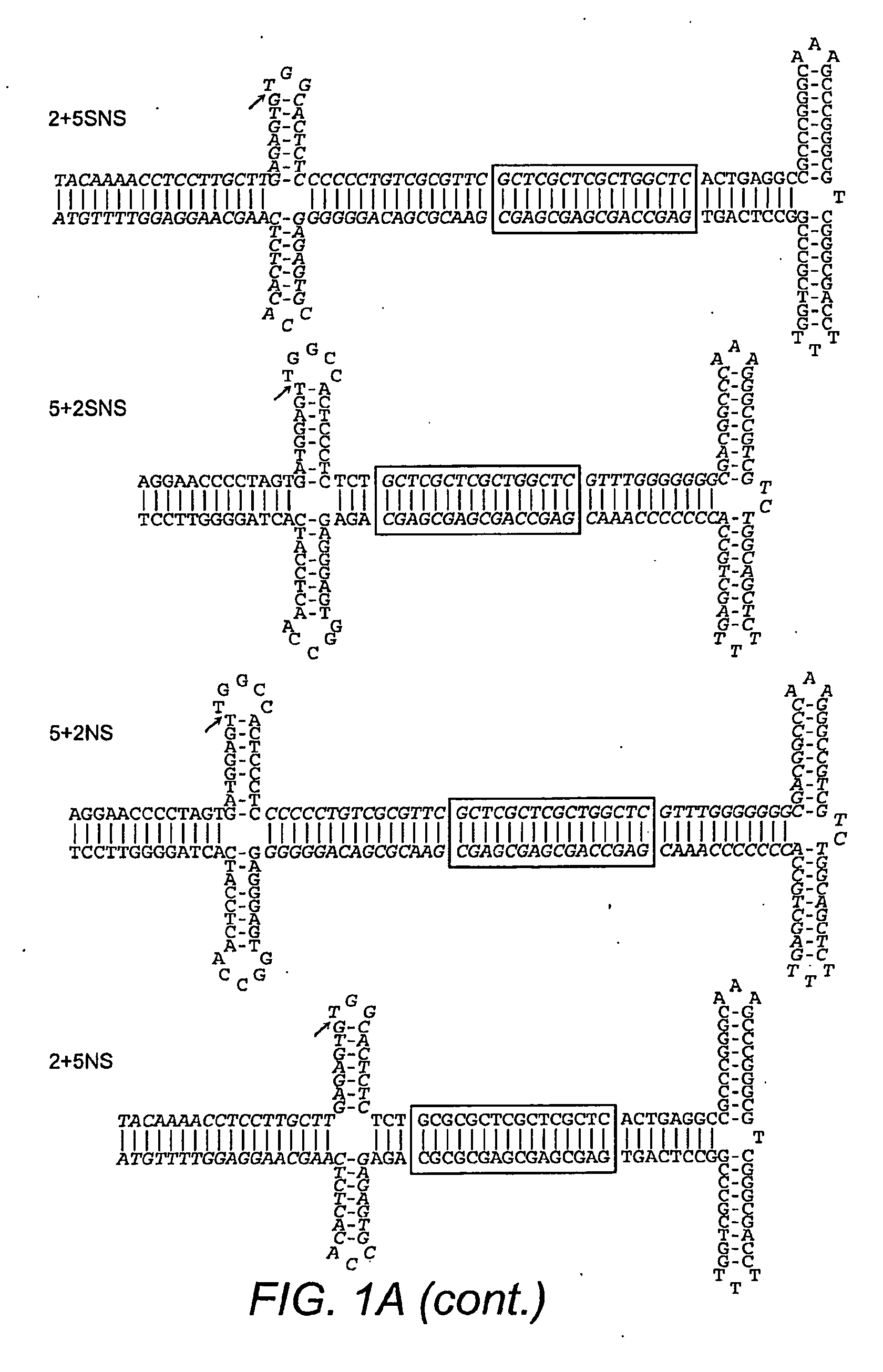
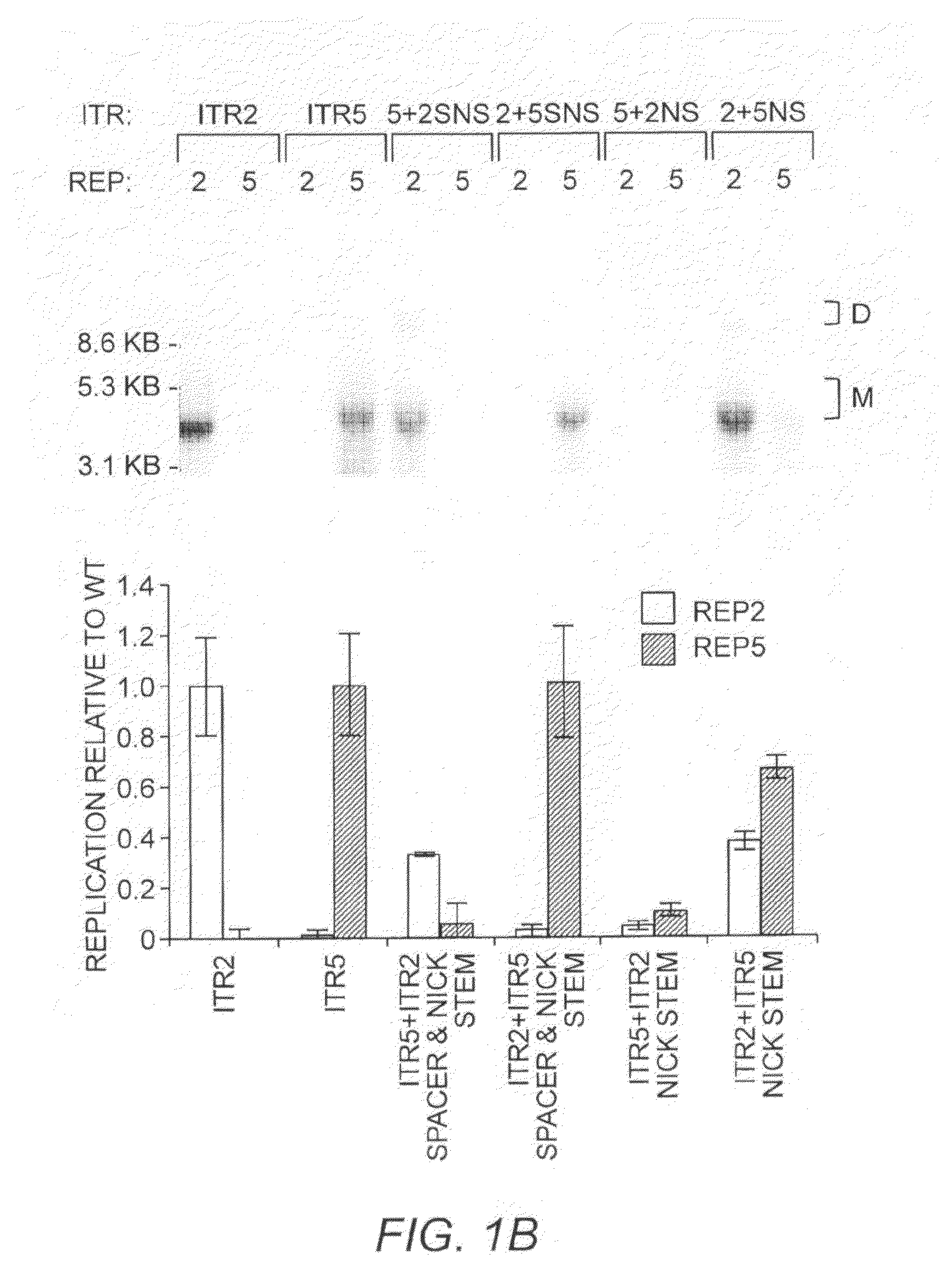
Restrictive inverted terminal repeats for viral vectors
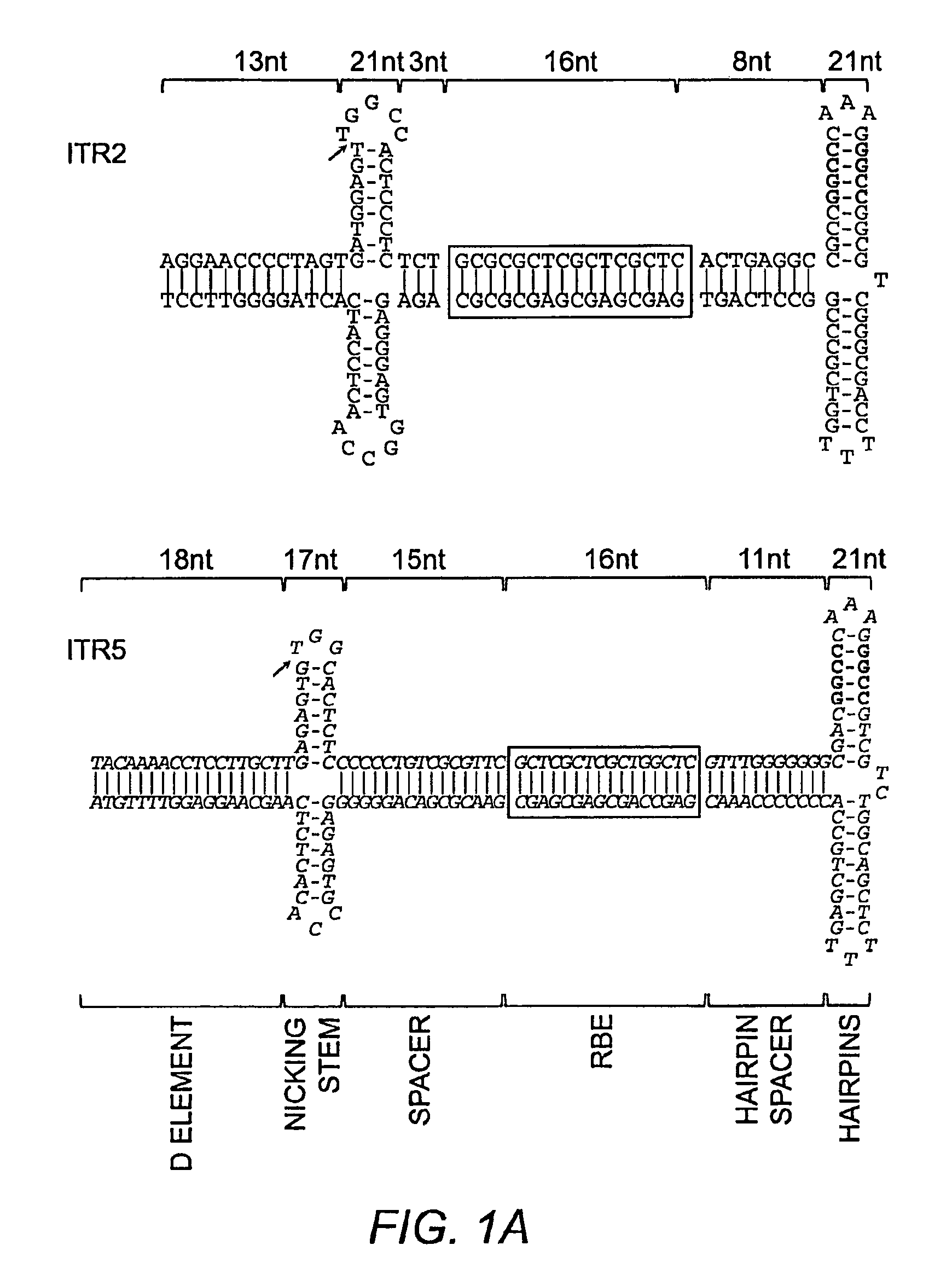
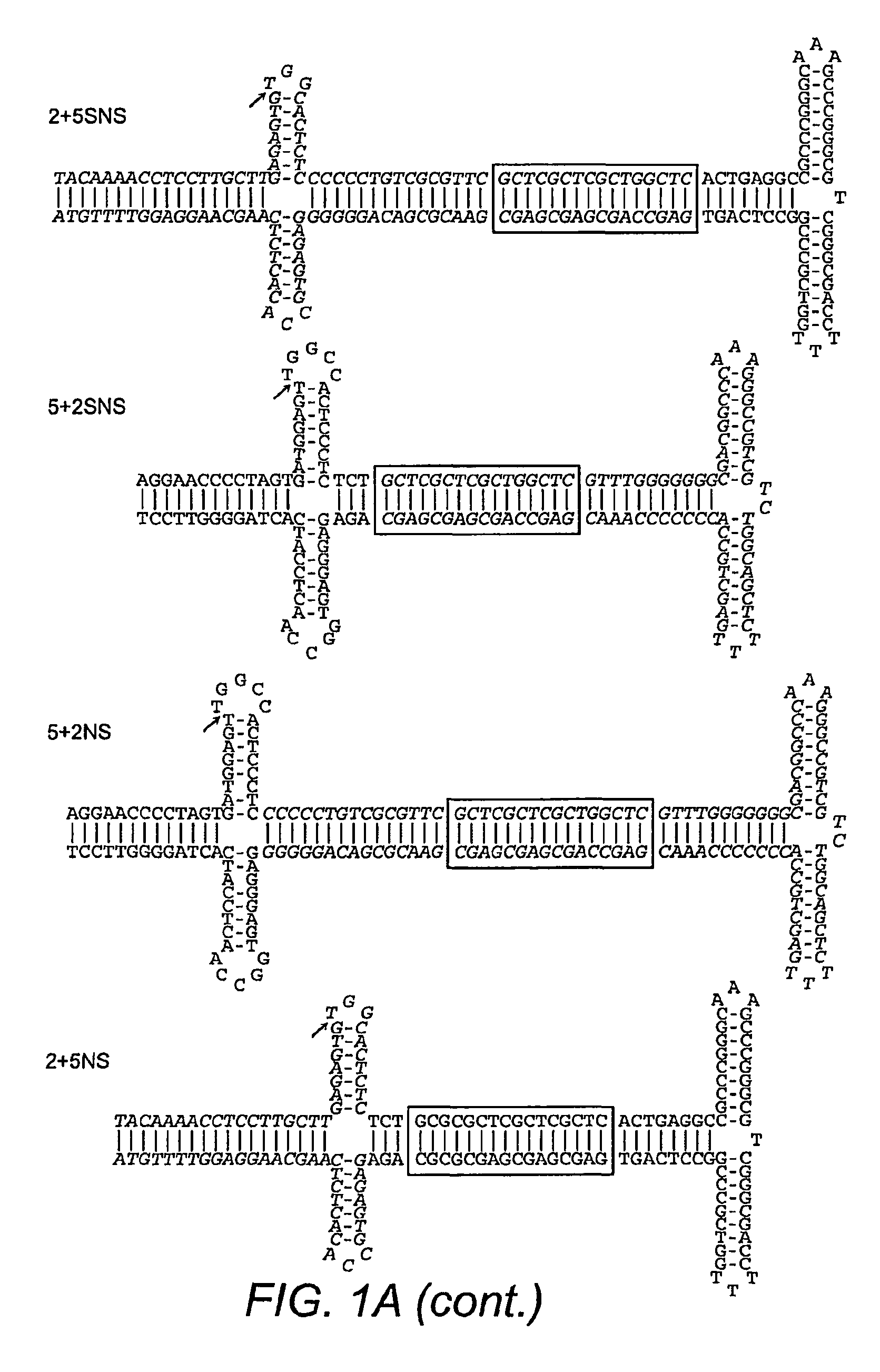
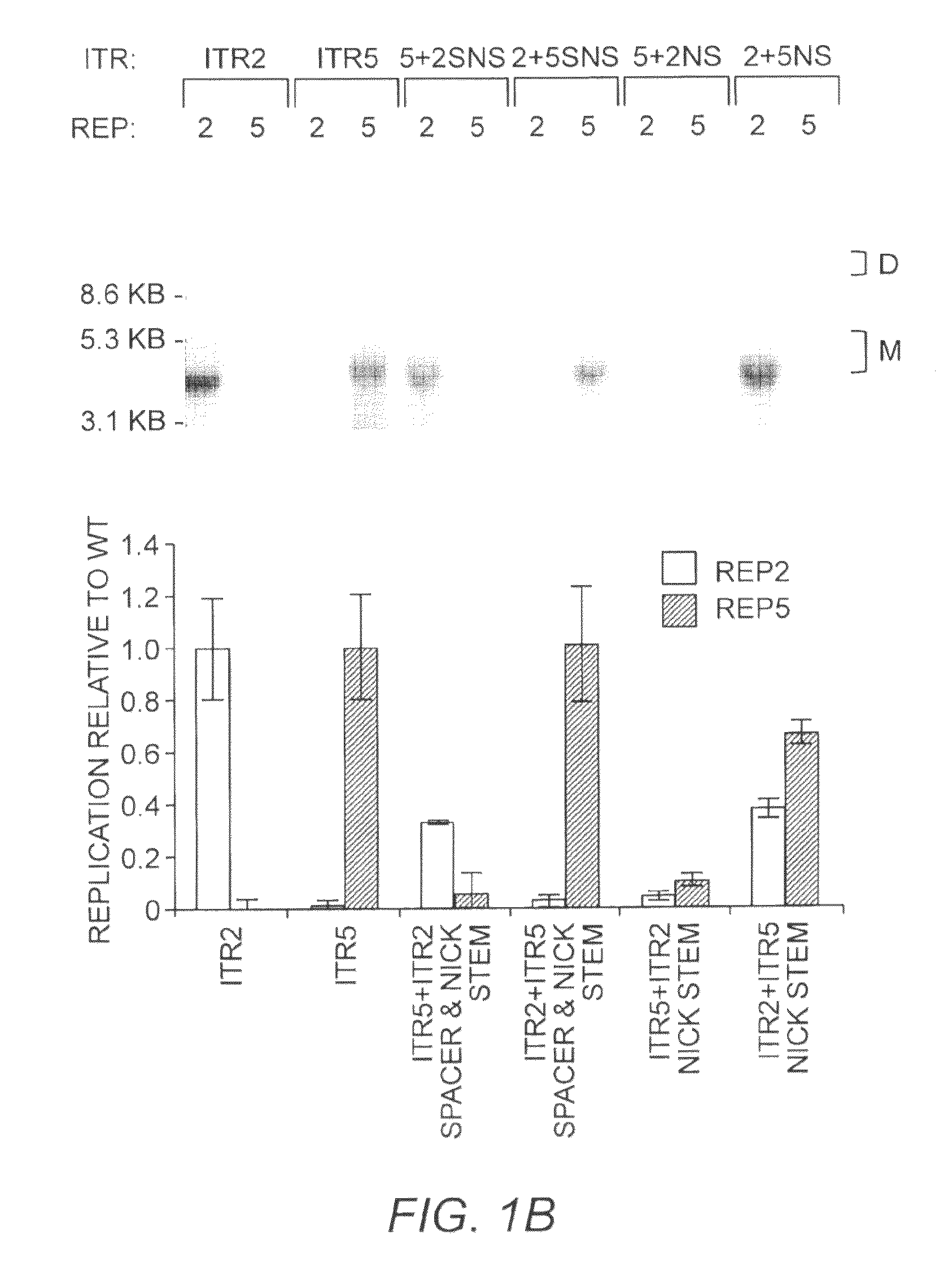
This invention relates to modified parvovirus inverted terminal repeats (ITRs) that do not functionally interact with wild-type large Rep proteins, synthetic Rep proteins that functionally interact with the modified ITRs, and methods of using the same for delivery of nucleic acids to a cell or a subject. The modifications provide a novel Rep-ITR interaction that limits vector mobilization, increasing the safety of viral vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
AAV vectors
InactiveUS6521225B1Prevent and limit expressionPrevents terminal glycosylation and translationBiocideOrganic active ingredientsLong terminal repeatNucleotide
The present invention is directed to a recombinant adenovirus vector comprising two inverted terminal repeats (ITRs) each of which comprises a D-sequence having (i) from 5 to 15 native nucleotides and (ii) one or more deletions or substitutions therein.
Owner:ADVANCED RES TECH INST +1
Capsid-free aav vectors, compositions, and methods for vector production and gene delivery
ActiveUS20140107186A1High-efficiency transductionEfficient and convenient methodNervous disorderGenetic material ingredientsGene deliveryTherapeutic intent
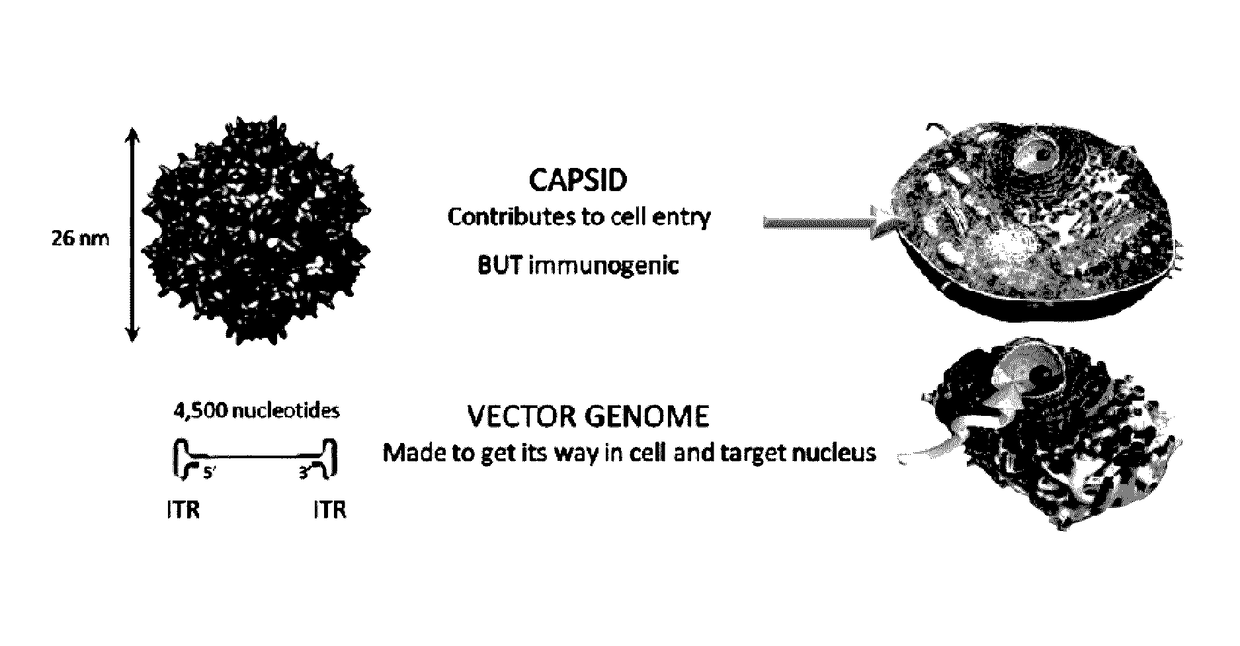
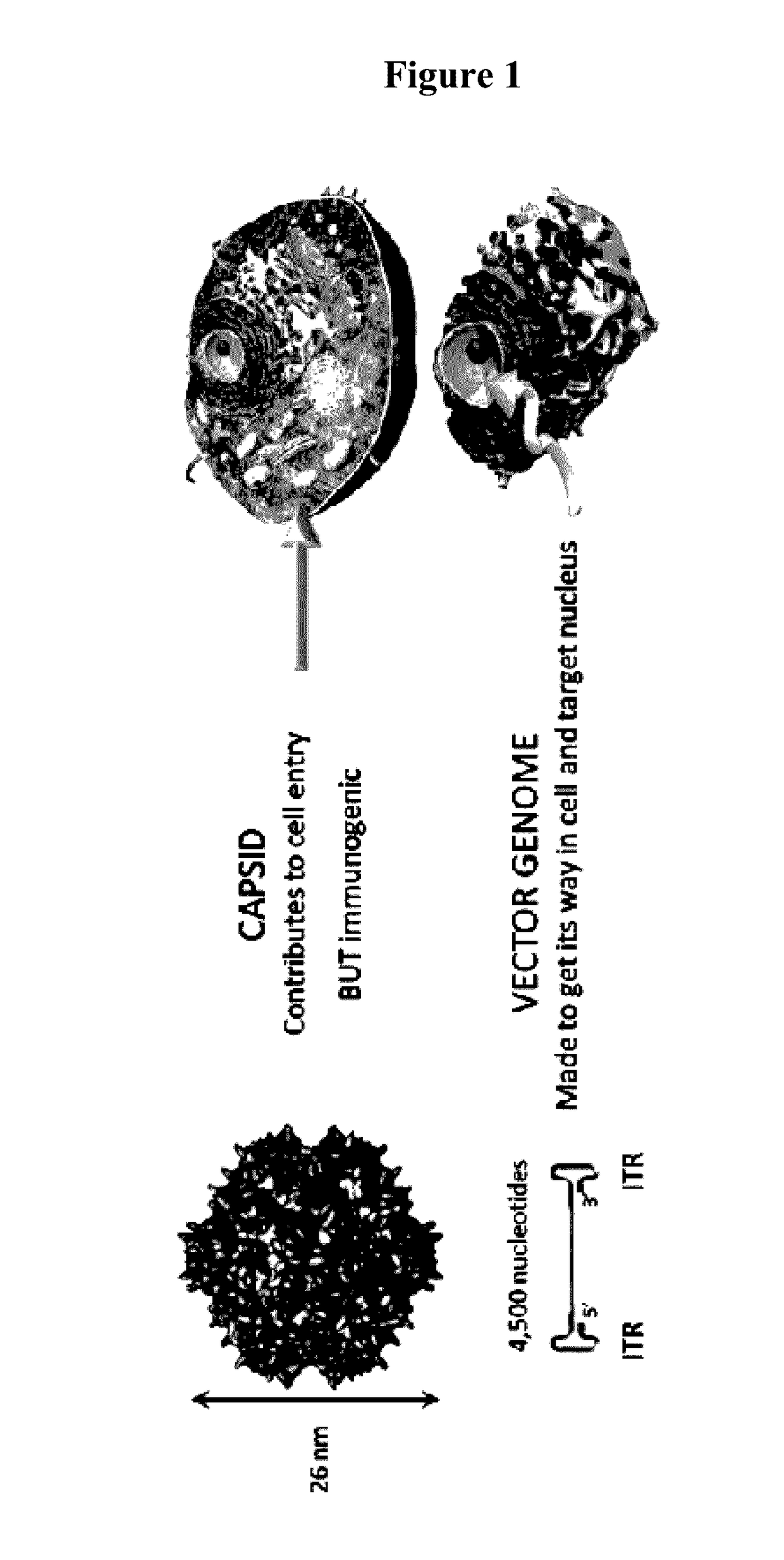
An isolated linear nucleic acid molecule comprising in this order: a first adeno-associated virus (AAV) inverted terminal repeat (ITR), a nucleotide sequence of interest and a second AAV ITR, wherein said nucleic acid molecule is devoid of AAV capsid protein coding sequences. The said nucleic acid molecule can be applied to a host at repetition without eliciting an immune response. Methods for producing and purifying this nucleic acid molecule, and use of the same for therapeutic purposes are also provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +4
Vectors with modified initiation codon for the translation of AAV-Rep78 useful for production of AAV
ActiveUS8512981B2Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorStart codonNucleotide
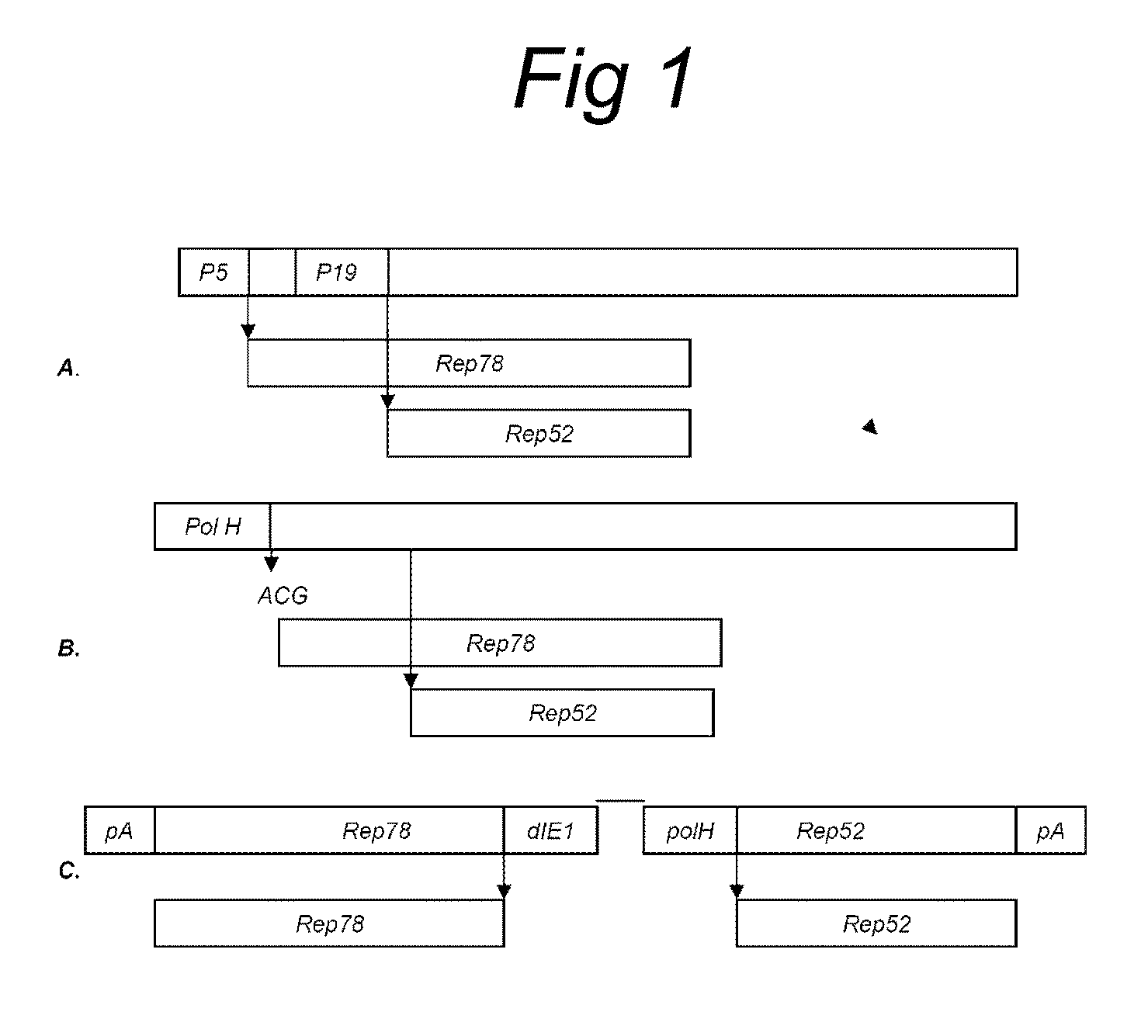
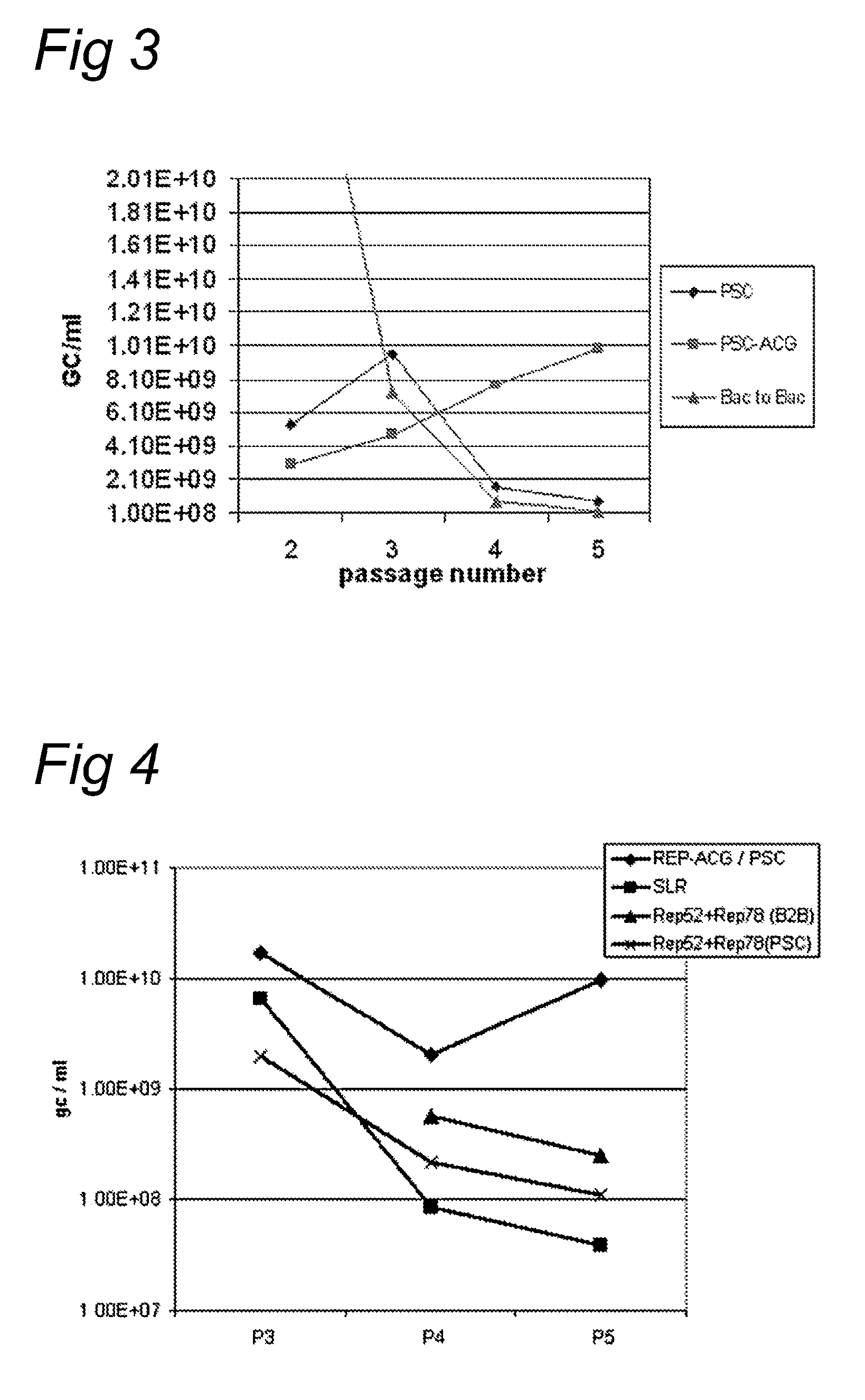
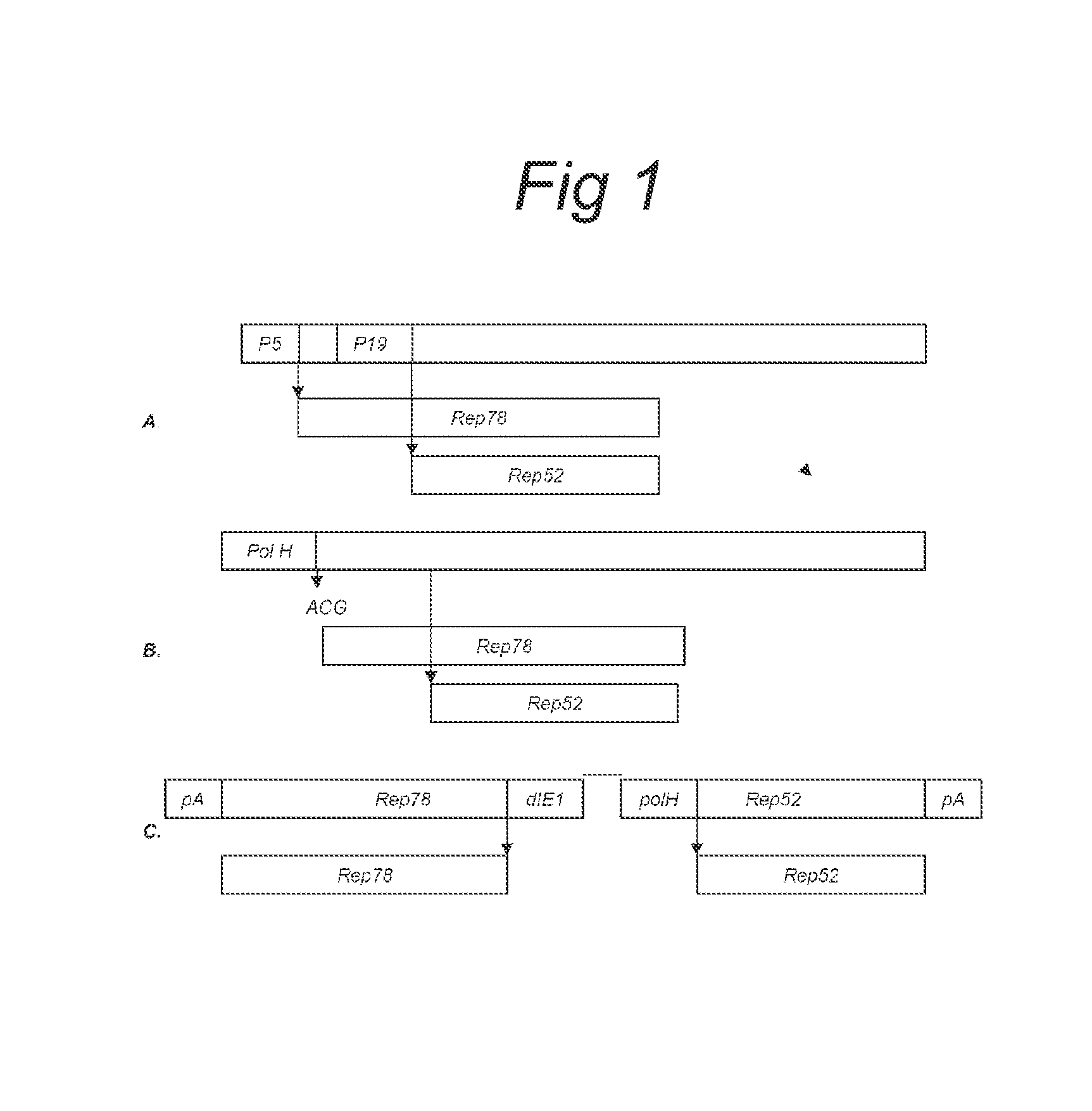
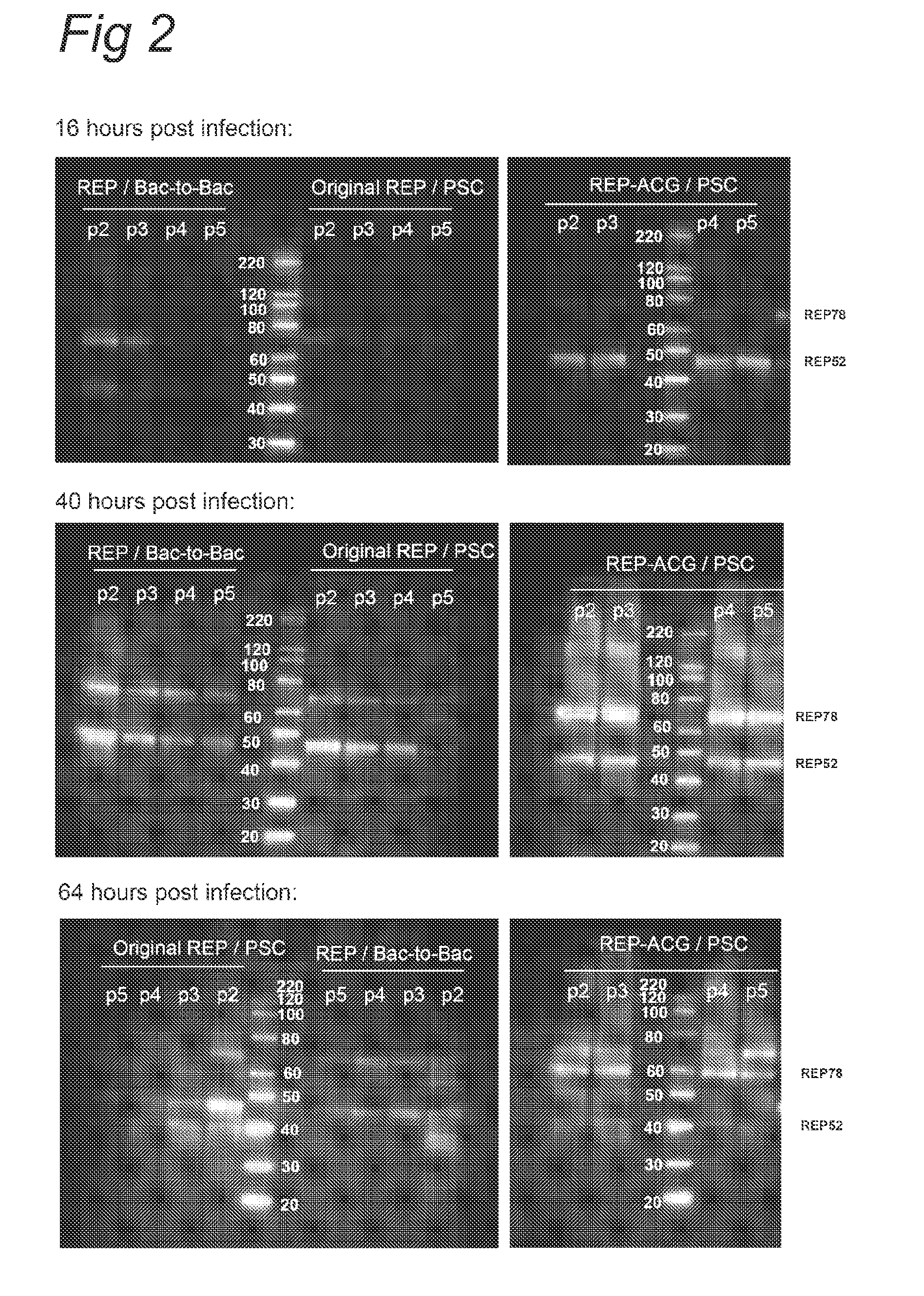
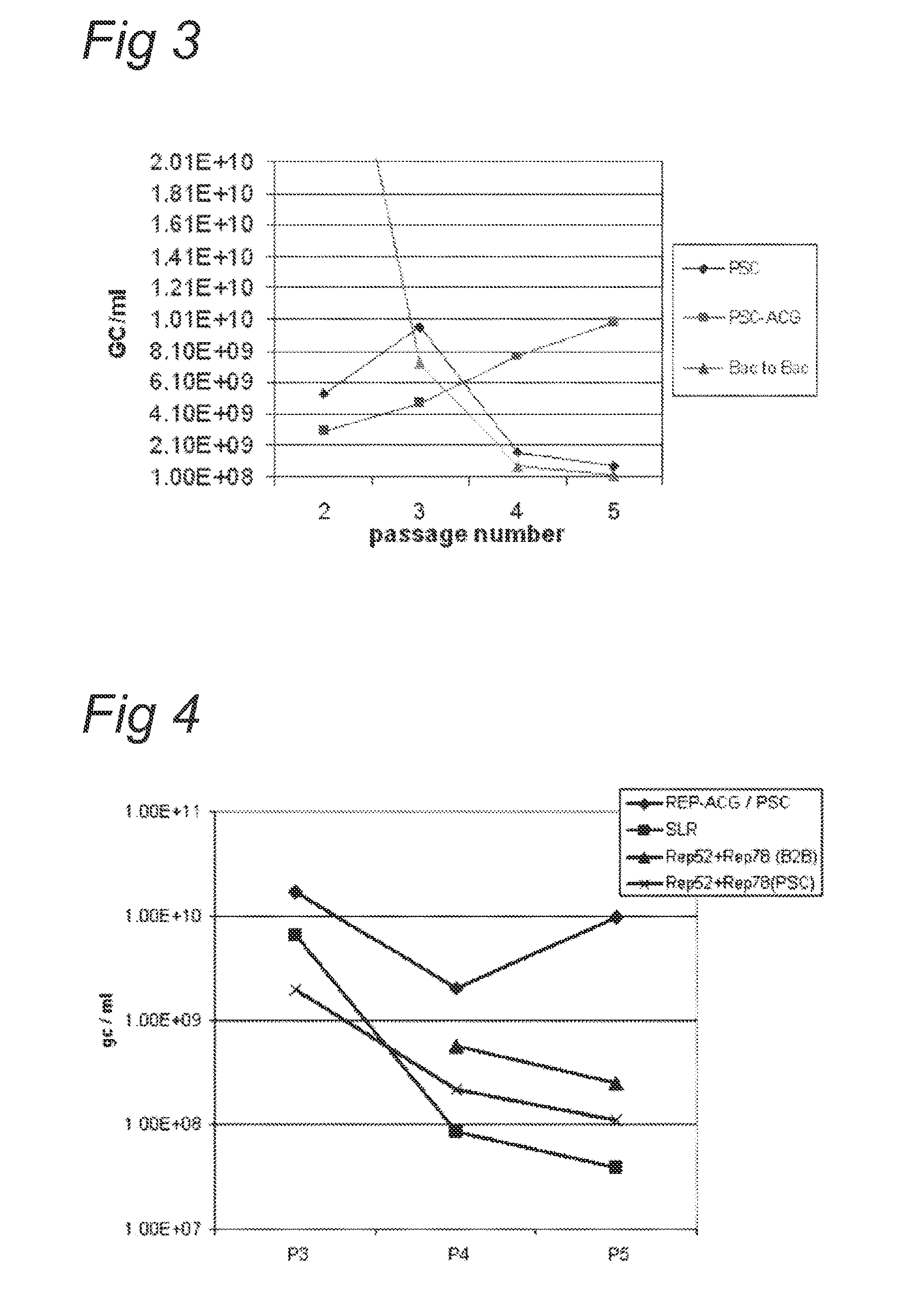
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Restrictive Inverted Terminal Repeats for Viral Vectors
This invention relates to modified parvovirus inverted terminal repeats (ITRs) that do not functionally interact with wild-type large Rep proteins, synthetic Rep proteins that functionally interact with the modified ITRs, and methods of using the same for delivery of nucleic acids to a cell or a subject. The modifications provide a novel Rep-ITR interaction that limits vector mobilization, increasing the safety of viral vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Viral vectors and methods for producing and using the same
InactiveUS20050220766A1Improve AAV production titerReduce and even essentially eliminate contaminationBiocidePeptide/protein ingredientsPolymerase LNucleic acid sequencing
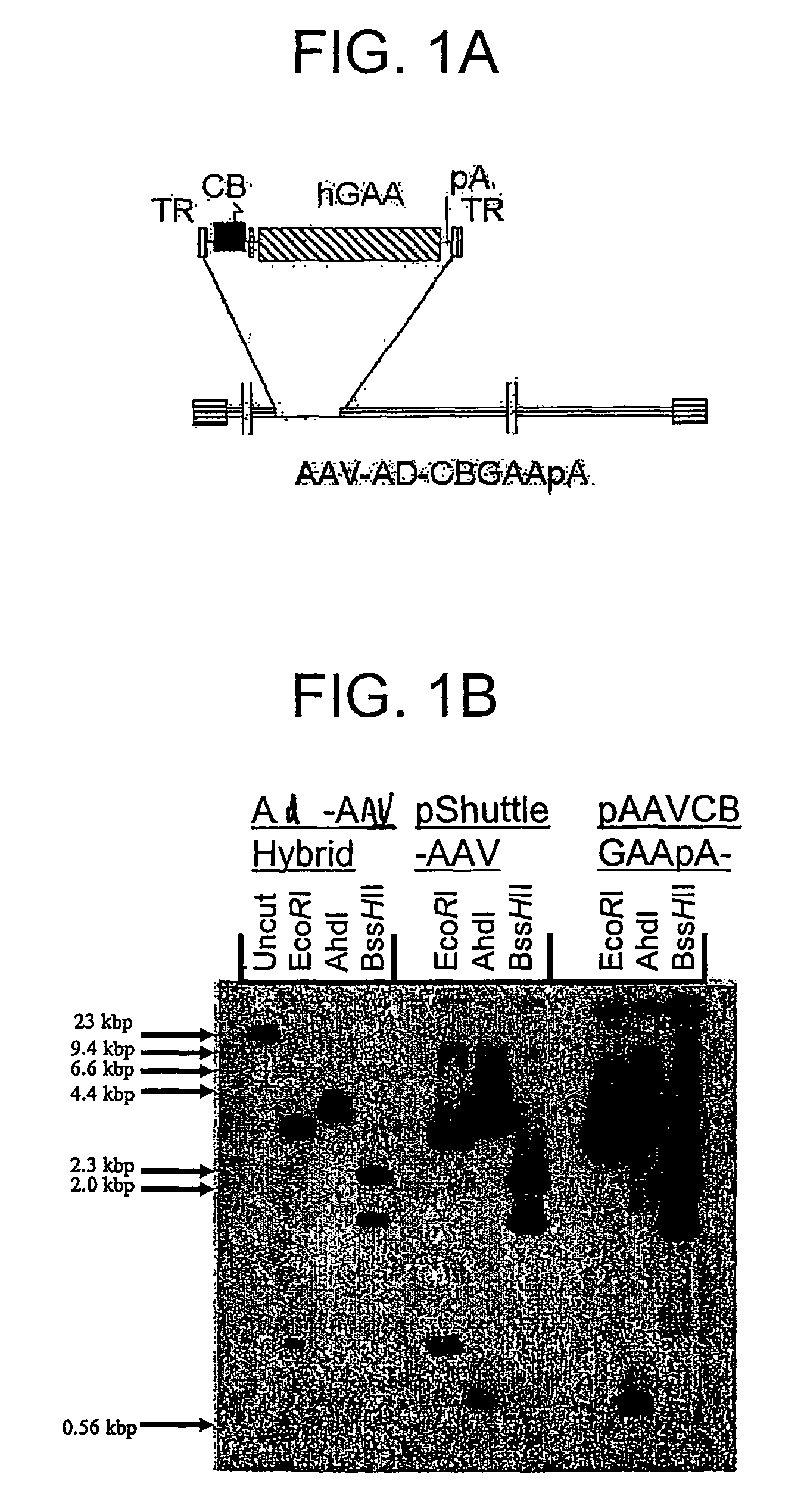
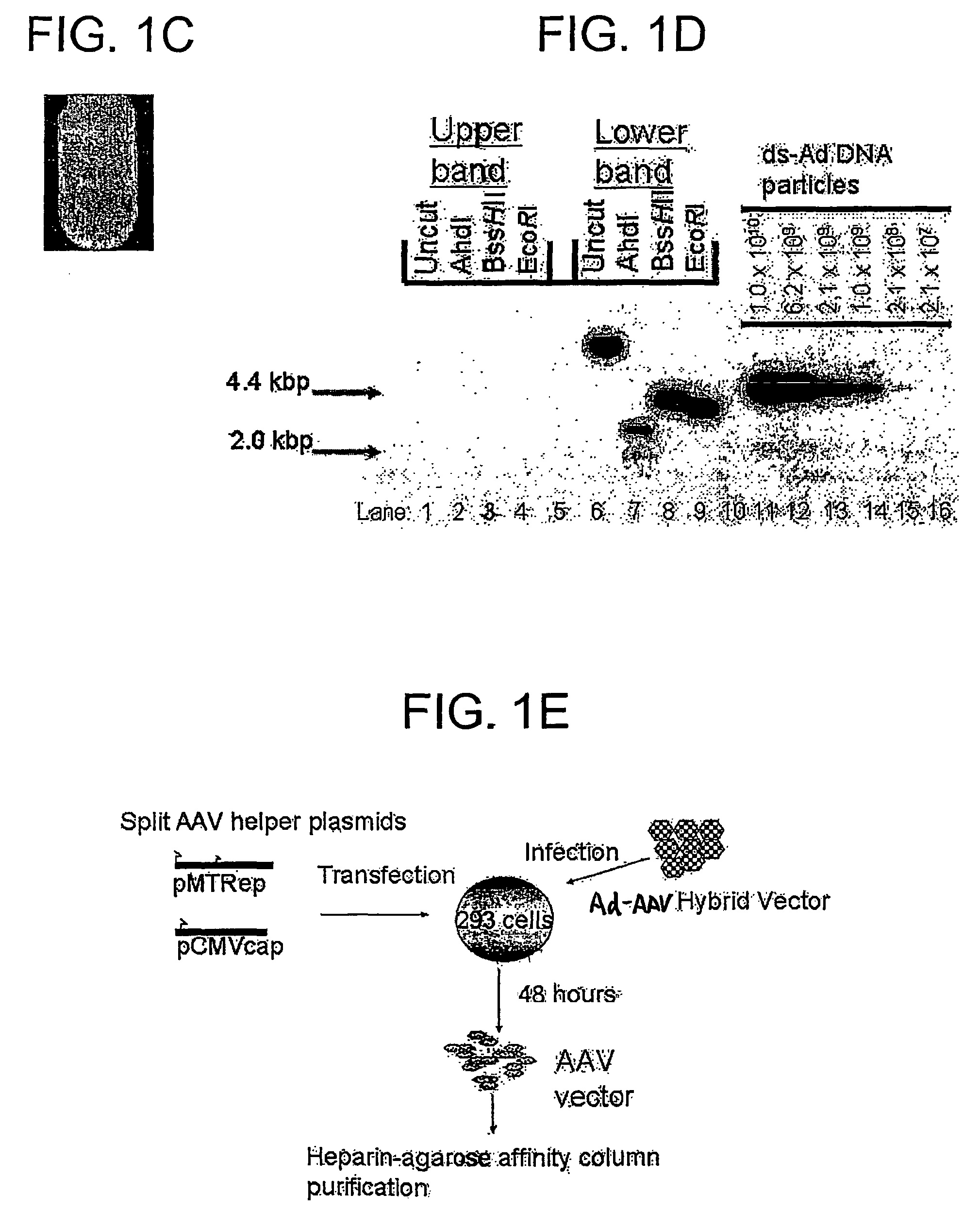
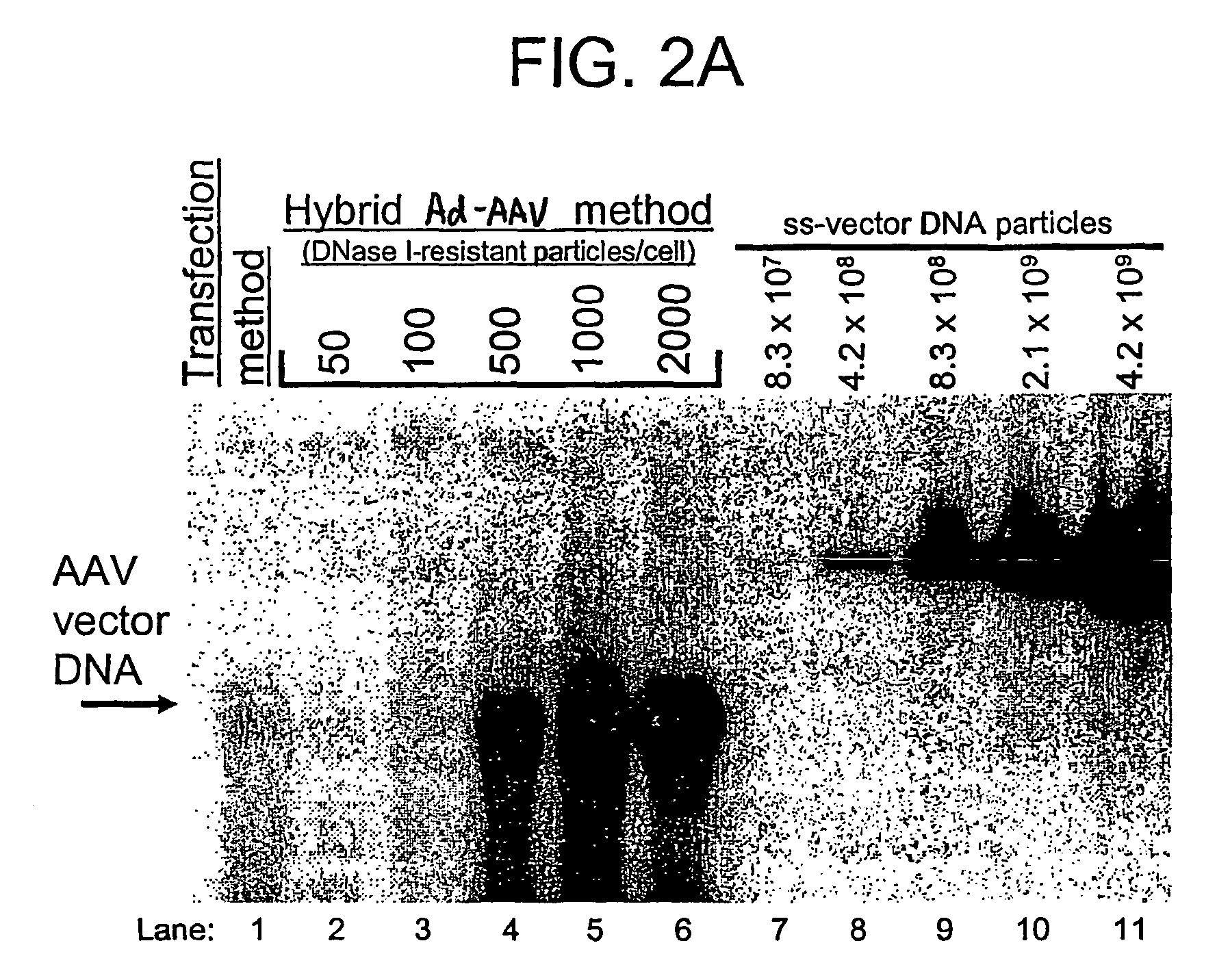
A recombinant hybrid virus, including: (a) a deleted adenovirus vector genome comprising the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation, and further comprising a deletion in an adenovirus genomic region selected from the group consisting of: (i) the polymerase region, wherein said deletion essentially prevents the expression of a functional polymerase protein from said deleted region and said hybrid virus does not otherwise express a functional polymerase protein, (ii) the preterminal protein region, wherein said deletion essentially prevents the expression of a functional preterminal protein from said deleted region, and said hybrid virus does not otherwise express a functional preterminal protein, and (iii) both the regions of (i) and (ii); and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), said recombinant AAV vector genome comprising (i) AAV 5′ and 3′ inverted terminal repeats, (ii) an AAV packaging sequence, and (iii) a heterologous nucleic acid sequence, wherein said heterologous nucleic acid sequence is flanked by the 5′ and the 3′ AAV inverted terminal repeats of (i). Methods of making and using the recombinant hybrid virus are also disclosed.
Owner:DUKE UNIV
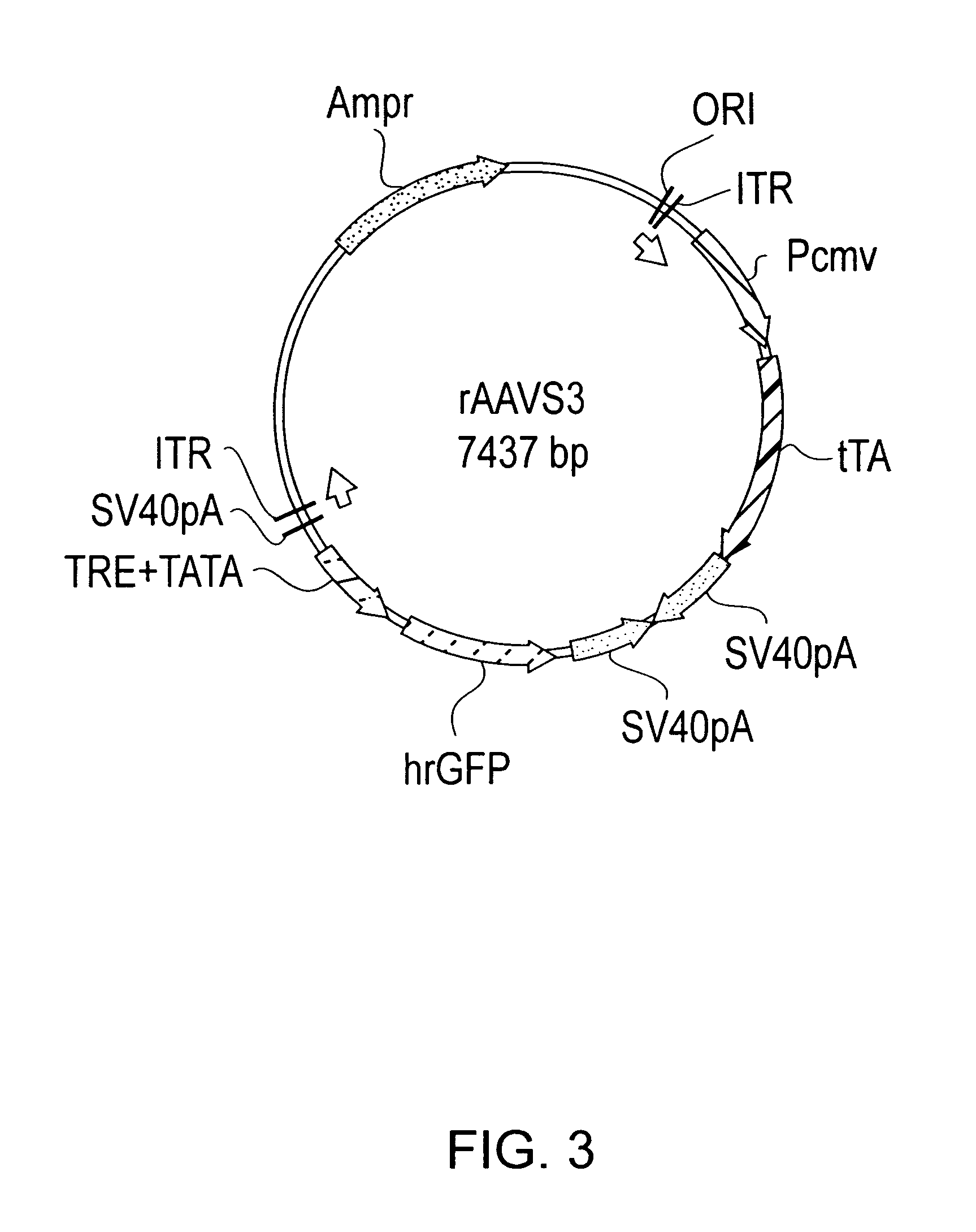
Tetracycline-regulated adeno-associated viral (AAV) vectors for gene delivery to the nervous system
Owner:CHILDRENS MEMORIAL HOSPITAL
Capsid-free AAV vectors, compositions, and methods for vector production and gene delivery
ActiveUS9598703B2Efficient and convenient methodElectrotherapyNervous disorderGene deliveryTherapeutic intent
An isolated linear nucleic acid molecule comprising in this order: a first adeno-associated virus (AAV) inverted terminal repeat (ITR), a nucleotide sequence of interest and a second AAV ITR, wherein said nucleic acid molecule is devoid of AAV capsid protein coding sequences. The said nucleic acid molecule can be applied to a host at repetition without eliciting an immune response. Methods for producing and purifying this nucleic acid molecule, and use of the same for therapeutic purposes are also provided.
Owner:UNITED STATES OF AMERICA +4
Methods of generating chimeric adenoviruses and uses for such chimeric aden oviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
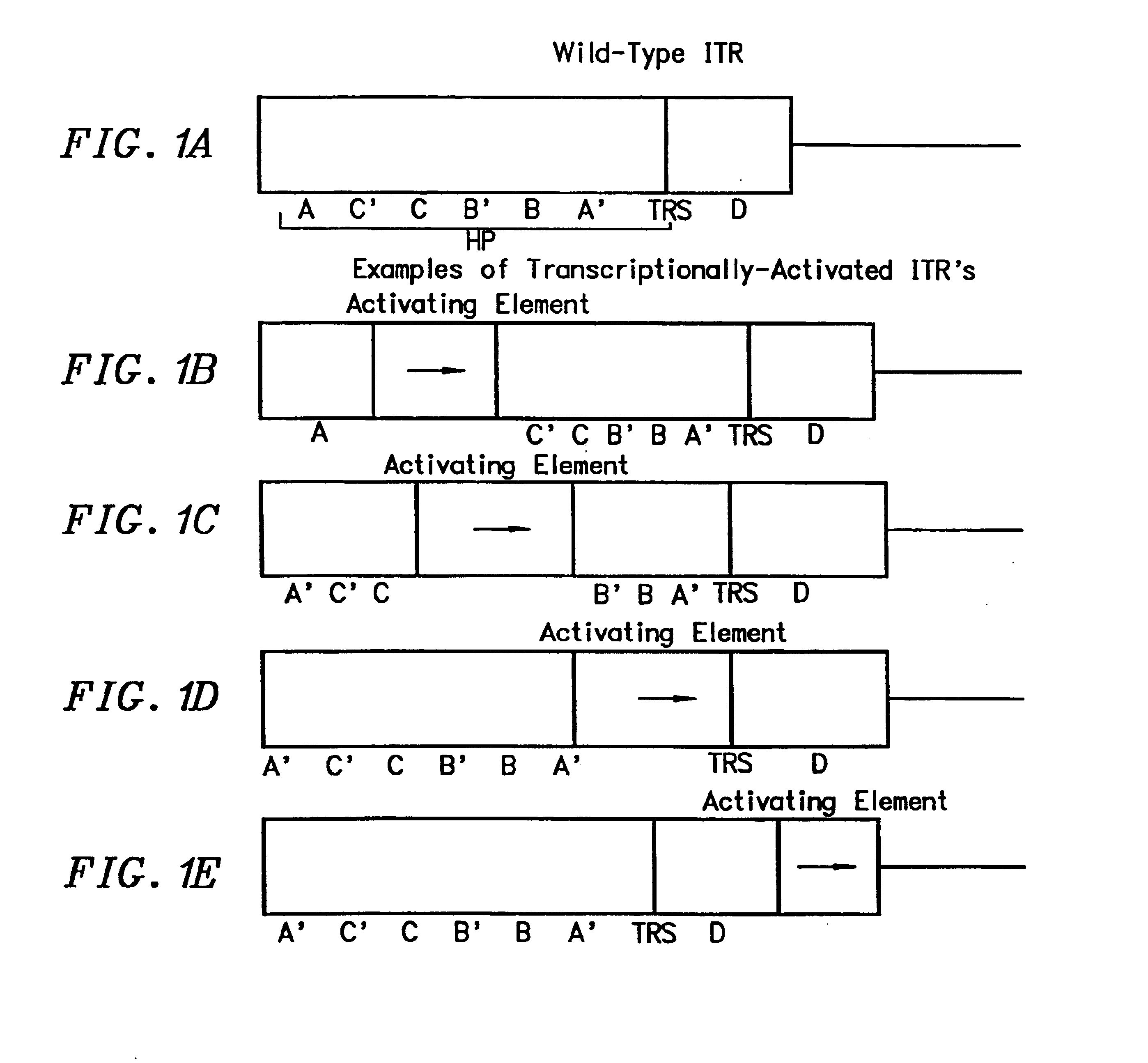
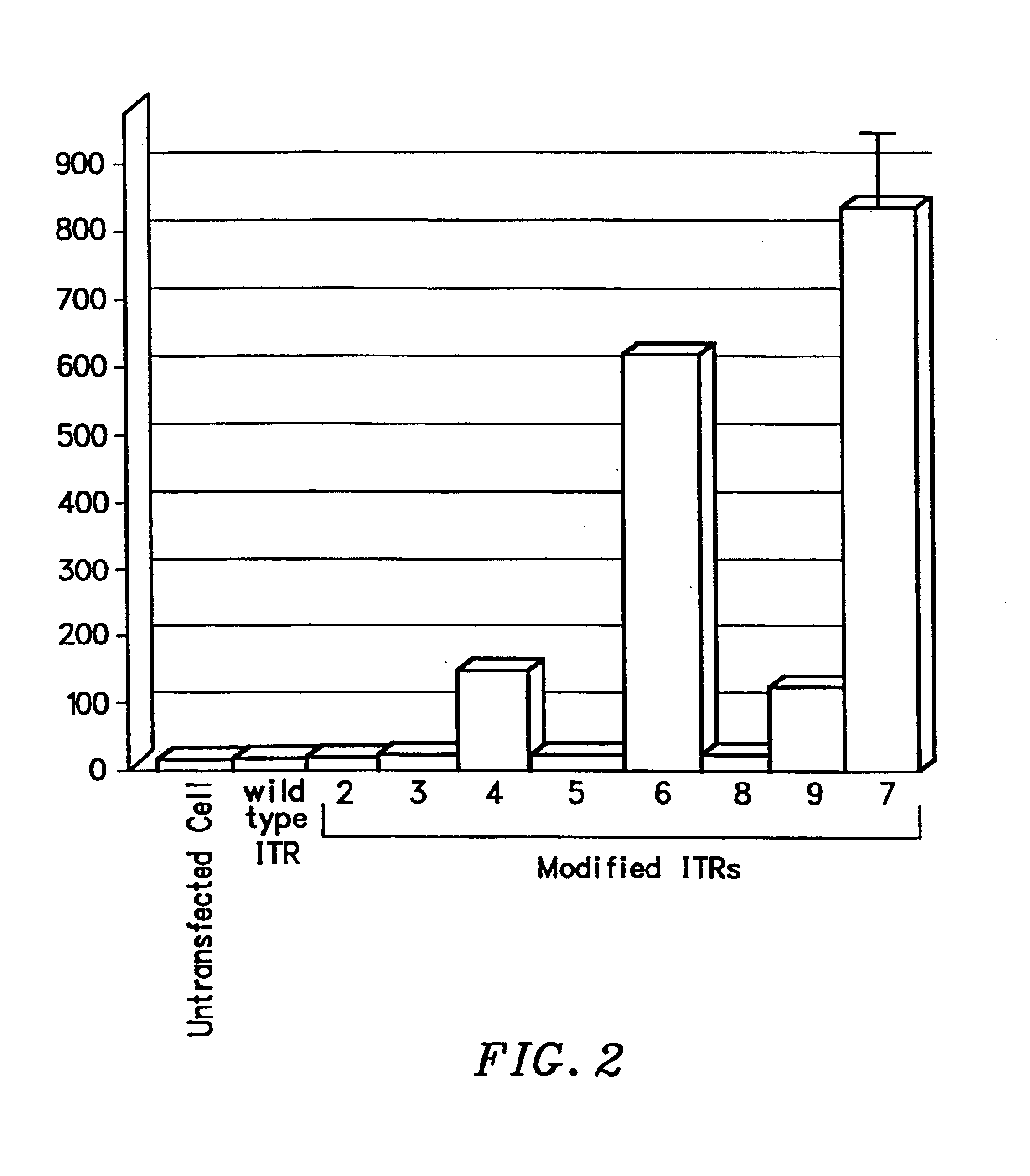
Transcriptionally-activated AAV inverted terminal repeats (ITRs) for use with recombinant AAV vectors
InactiveUS6936466B2Increase transcriptional activityGenetic material ingredientsFermentationLong terminal repeatGenetics
This invention provides transcriptionally-activated AAV ITRs (inverted terminal repeats) which are small and transcriptionally active and uses thereof to optimize the expression of relatively large transgenes packaged in recombinant AAV vectors.
Owner:TARGETED GENETICS CORPORTION
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20130296532A1Improve stabilityReduced expression levelVirus peptidesNucleic acid vectorNucleotideCapsid
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:UNIQURE IP BV
Viral vectors and methods for producing and using the same
InactiveUS7858367B2Reduce and even essentially eliminate contaminationReduce dependenceBiocidePeptide/protein ingredientsHeterologousPolymerase L
A recombinant hybrid virus which includes: (a) a deleted adenovirus vector genome having the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation and a deletion in an adenovirus genomic region selected from the polymerase region and / or the preterminal protein region, wherein the deletion essentially prevents the expression of a functional polymerase and / or preterminal protein from the deleted region and the hybrid virus does not otherwise express a functional polymerase protein; and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), wherein the recombinant AAV vector genome includes an AAV packaging sequence and a heterologous nucleic acid sequence, wherein the heterologous nucleic acid sequence is flanked by 5′ and 3′ AAV inverted terminal repeats.
Owner:DUKE UNIV
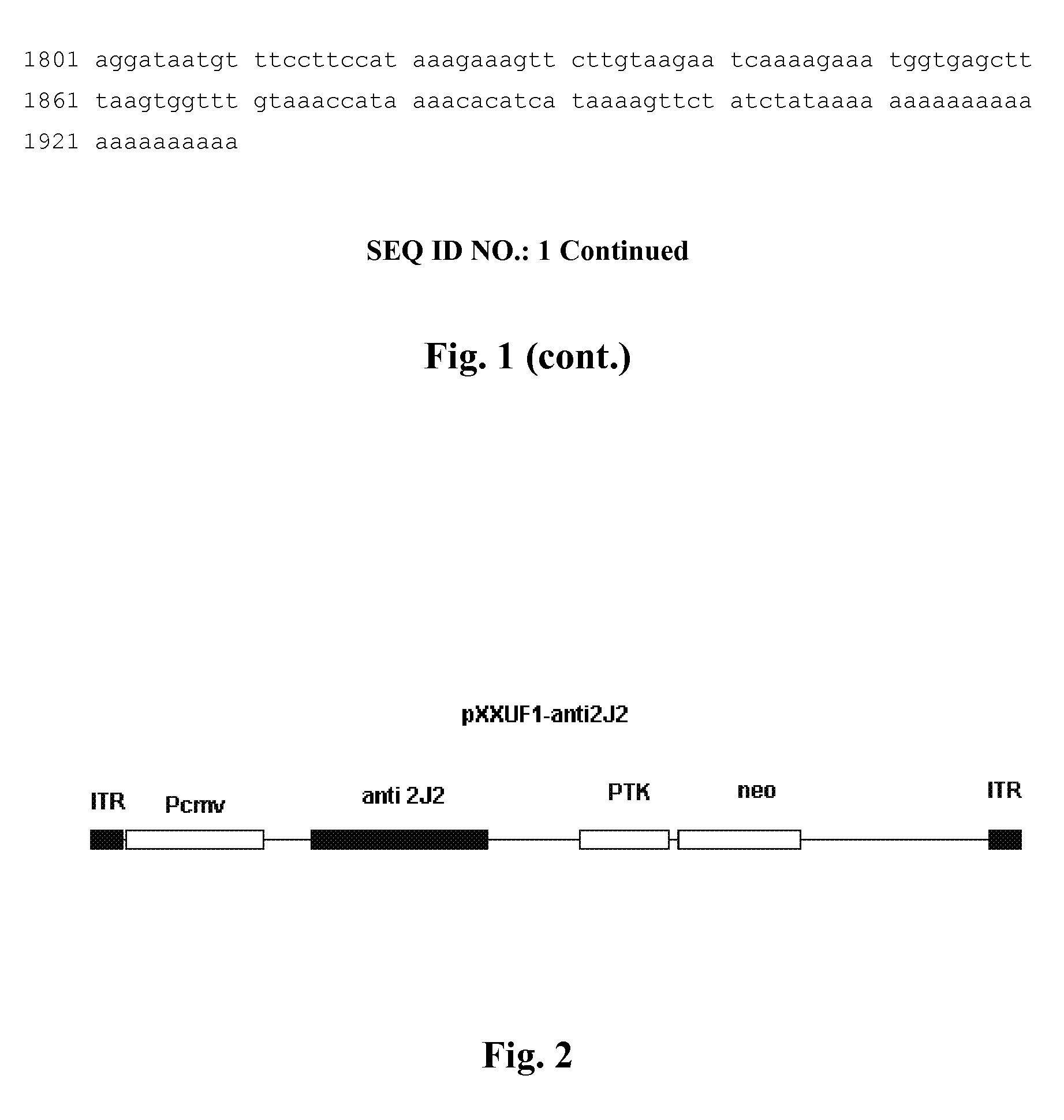
Recombinant adeno-associated virus expressing human antisense gene CyP2J2 and its preparation methods
ActiveUS8273344B2Inhibit proliferation and migrationPromote apoptosisBiocideGenetic material ingredientsNucleotideGenetics
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Constructs and Methods for Delivering Molecules via Viral Vectors with Blunted Innate Immune Responses
InactiveUS20140271550A1Increase gene expressionLower immune responseBiocideSugar derivativesNucleotideGene product
A CpG-modified recombinant adeno-associated viral (AAV) vector is described. The vector carries a nucleic acid molecule comprising AAV inverted terminal repeat (ITR) sequences and an exogenous gene sequence under the control of regulatory sequences which control expression of the gene product, in which the nucleic acid sequences carried by the vector are modified to significantly reduce CpG di-nucleotides such that an immune response to the vector is reduced as compared to the unmodified AAV vector. Also provided are methods and regimens for delivering transgenes using these AAV viral vectors, in which the innate immune response to the vector and / or transgene is significantly modulated.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Method for quantifying adeno-associated virus
ActiveUS20160273058A1Improve efficiencyMicrobiological testing/measurementDNA/RNA fragmentationAdeno associate virusNucleotide sequencing
A method for quantification of an adeno-associated virus genome, including the steps of (a) preparing a composition containing a sample, at least one primer pair for use in amplification of only a nucleotide sequence contained in inverted terminal repeats of an adeno-associated virus, and an intercalating dye; (b) performing nucleic acid amplification reaction using the composition prepared in the step (a); and (c) detecting an amplified product obtained in the step (b). The present invention is especially useful in the fields of medicine, gene engineering, and biology.
Owner:TAKARA HOLDINGS
Vectors with modified initiation codon for the translation of aav-rep78 useful for production of aav
ActiveUS20090191588A1Improve stabilityReduced expression levelAnimal cellsSugar derivativesStart codonNucleotide
The present invention relates nucleic acid constructs for the production of recombinant parvoviral (e.g. adeno-associated viral) vectors in insect cells, to insect cells comprising such constructs and to methods wherein the cells are used to produce recombinant parvoviral virions. The insect cells preferably comprise a first nucleotide sequence encoding the parvoviral rep proteins whereby the initiation codon for translation of the parvoviral Rep78 protein is a suboptimal initiation codon that effects partial exon skipping upon expression in insect cells. The insect cell further comprises a second nucleotide sequence comprising at least one parvoviral (AAV) inverted terminal repeat (ITR) nucleotide sequence and a third nucleotide sequence comprising a sequences coding for the parvoviral capsid proteins.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS +1
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
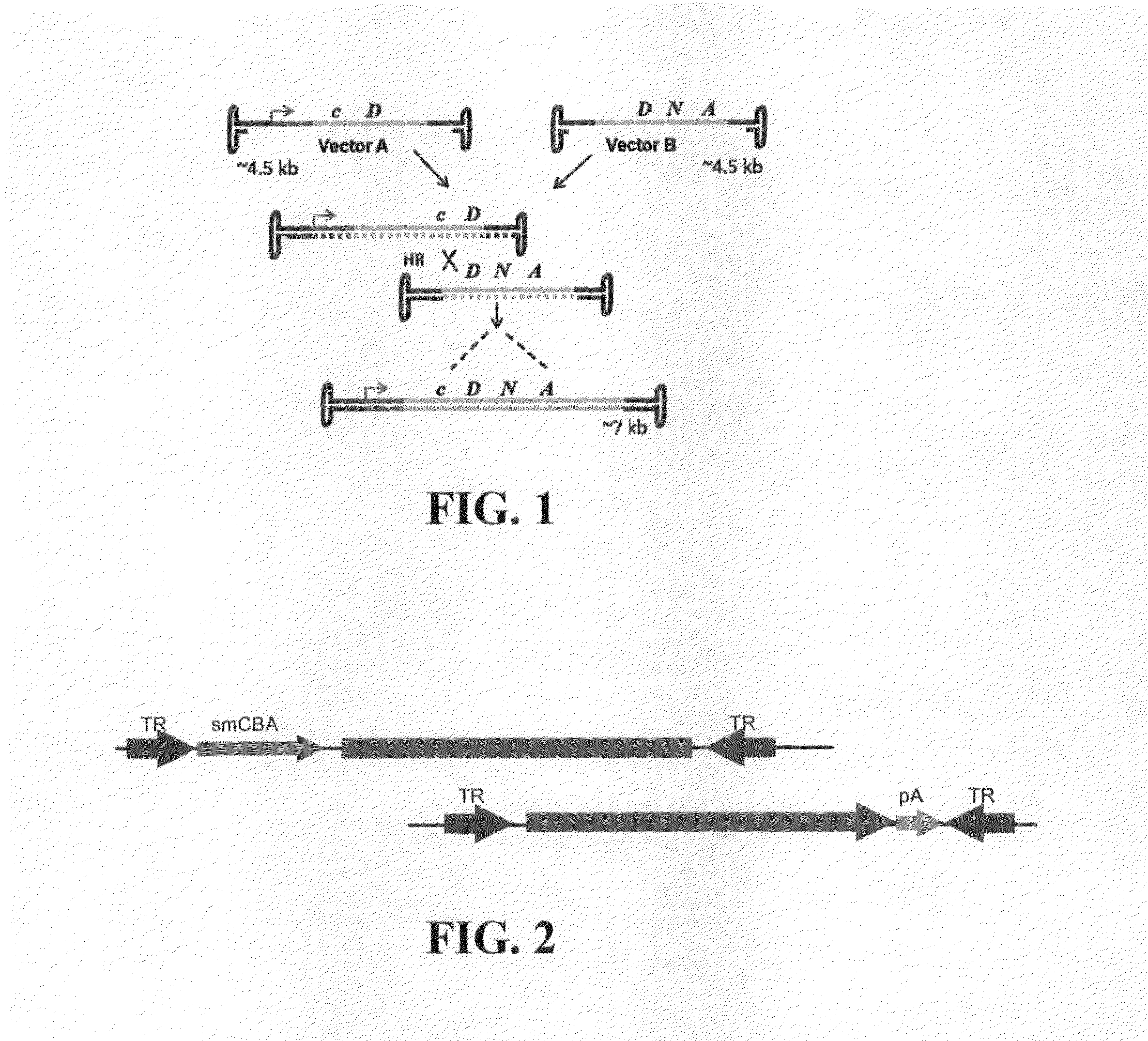
Dual-AAV Vector-Based Systems and Methods for Delivering Oversized Genes to Mammalian Cells
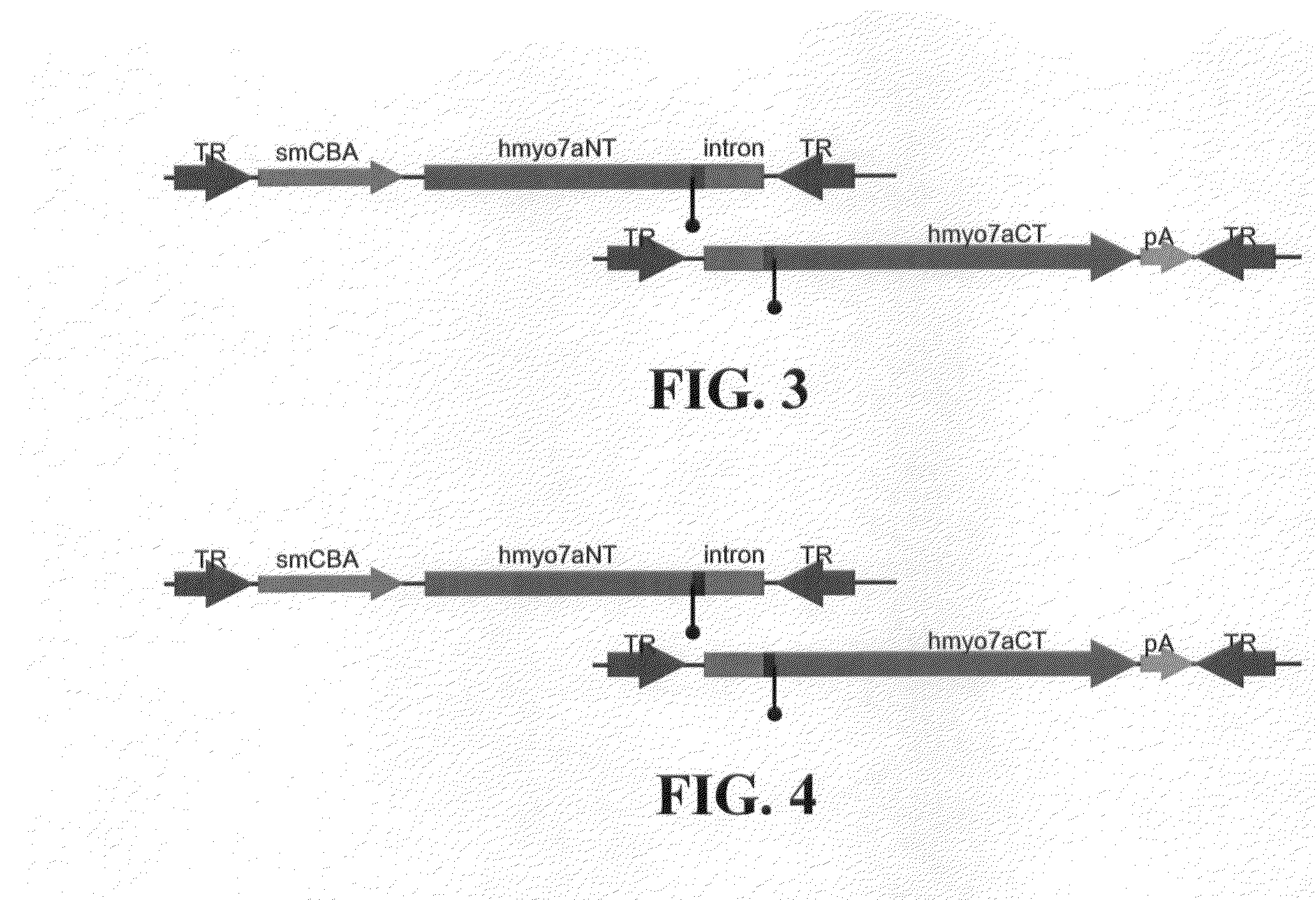
Disclosed are materials and methods for treating diseases of the mammalian eye, and in particular, Usher syndrome 1B (USH1B). The invention provides AAV-based, dual-vector systems that facilitate the expression of full-length proteins whose coding sequences exceed that of the polynucleotide packaging capacity of an individual AAV vector. In one embodiment, vector systems are provided that include i) a first AAV vector polynucleotide that includes an inverted terminal repeat at each end of the polynucleotide and a suitable promoter followed by a partial coding sequence that encodes an N-terminal portion of a full-length polypeptide; and ii) a second AAV vector polynucleotide that includes an inverted terminal repeat at each end of the polynucleotide and a partial coding sequence that encodes a C-terminal portion of a full-length polypeptide, optionally followed by a polyadenylation (pA) signal sequence. In another embodiment, the vector system includes i) a first AAV vector polynucleotide comprising an inverted terminal repeat at each end, a suitable promoter followed by a partial coding sequence that encodes an N-terminal portion of a full-length polypeptide followed by a splice donor site and intron and ii) a second AAV vector polynucleotide comprising an inverted terminal repeat at each end, followed by an intron and a splice-acceptor site for the intron, followed by a partial coding sequence that encodes a C-terminal portion of a full-length polypeptide, optionally followed by a polyadenylation (pA) signal sequence. The coding sequence or the intron sequence in the first and second AAV vectors preferably includes a sequence region that overlaps.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Generation of recombinant adeno-associated viral vectors by a complete adenovirus-mediated approach
A complete virus-mediated system for recombinant AAV production including: (a) a first recombinant adenovirus encoding one or more AAV REP78 / 68 polypeptides and one or more viral capsid polypeptides; (b) a second recombinant adenovirus encoding a gene of interest and including AAV inverted terminal repeats, wherein the AAV inverted terminal repeats flank the gene of interest; (c) viral helper functions; and (d) a host cell transduced with the first recombinant adenovirus, the second recombinant adenovirus, and the viral helper functions.
Owner:LI CHUAN YAN +1
Method for producing heart-specific fluorescence of non-human eukaryotic animals
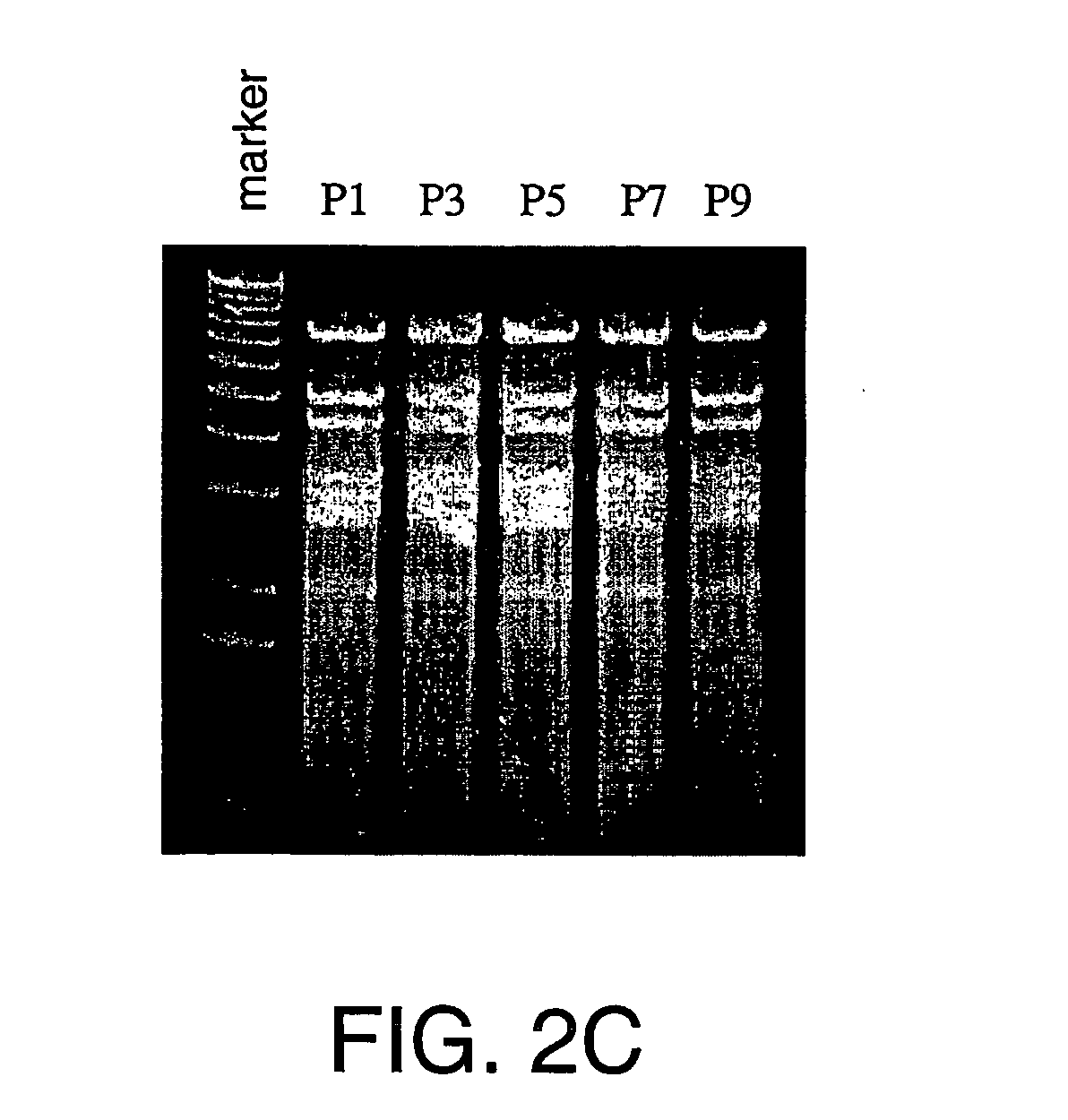
A method of expressing in vivo heart-specific fluorescence in transgenic line of zebrafish is developed, which provides a research model for studying heart-related gene functions and performing gene therapies in the future. The method comprises the following steps. Firstly, a plasmid is constructed. This plasmid construct includes the upstream regulatory region, the exon 1, the intron 1, and the exon 2 of cmlc2 gene, cDNA of GFP, wherein the cmlc2 gene and GFP cDNA form a cassette, and inverted terminal repeats from adeno-associated virus are flanked at both sides of this cassette. The plasmid construct is linearized and microinjected into one-celled zebrafish fertilized eggs. Lastly, the heart-specific fluorescent expressed zebrafish are selected and the germline-transmitting transgenic strain is generated.
Owner:NAT TAIWAN UNIV
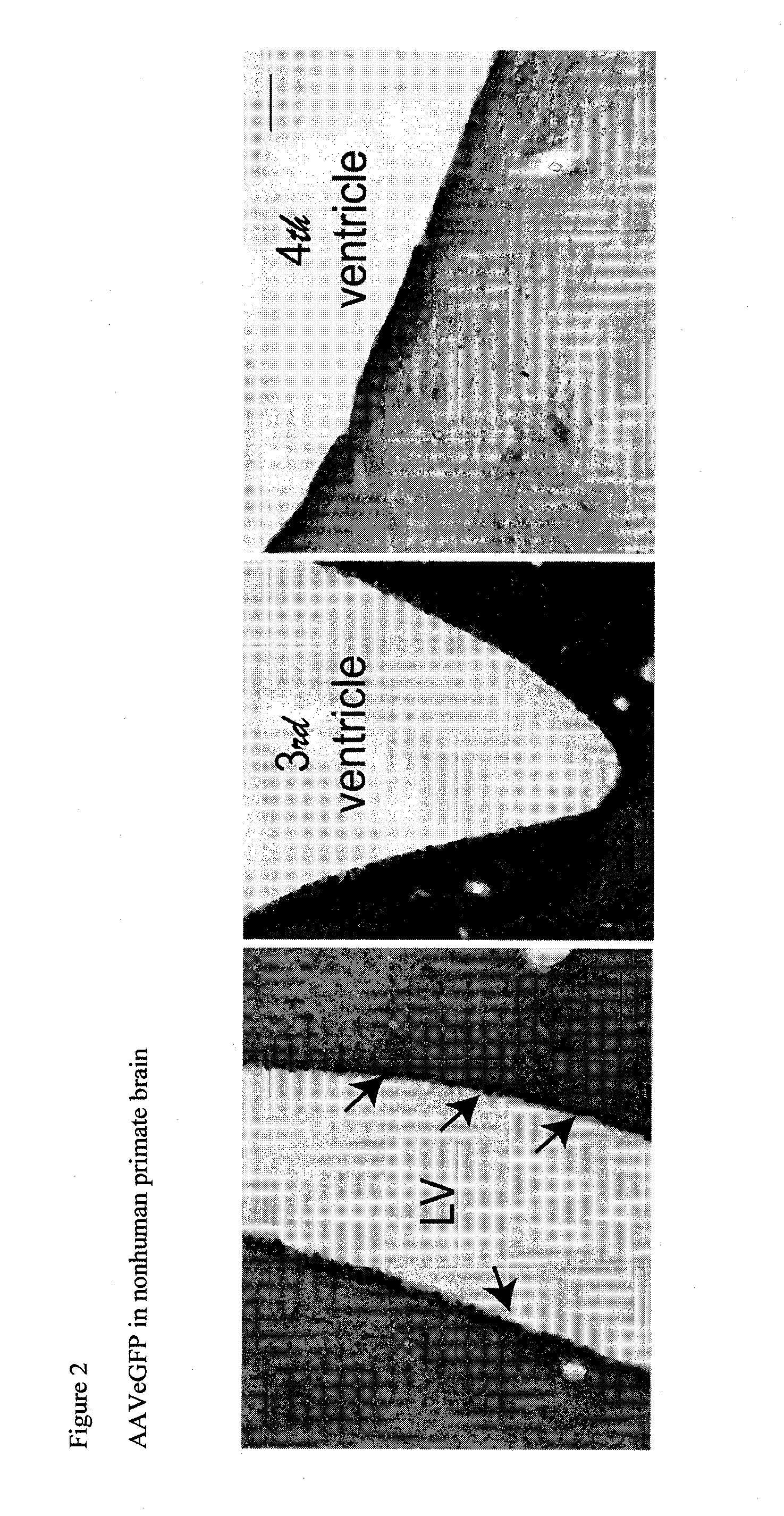
Methods and compositions for treating brain diseases
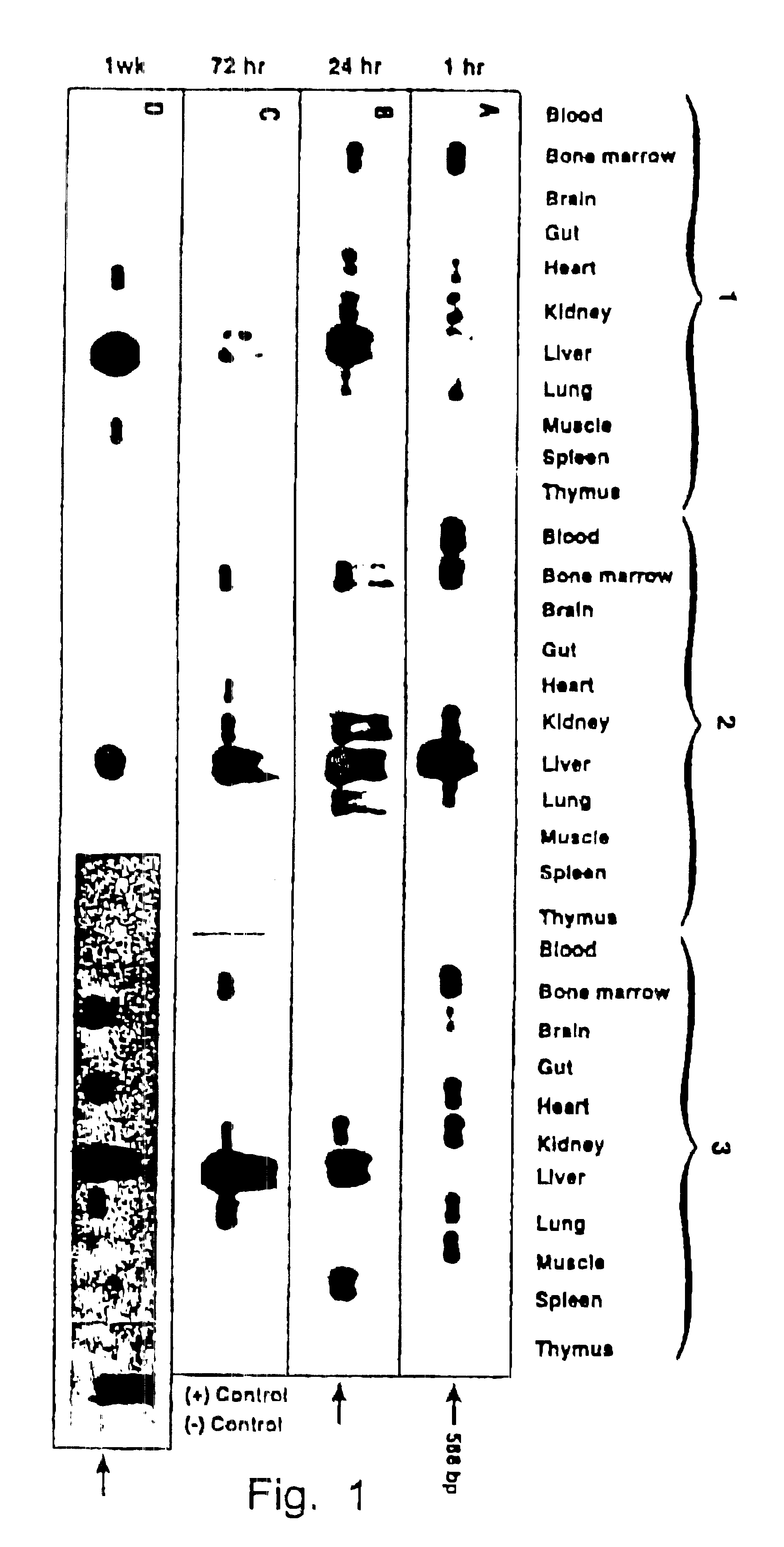
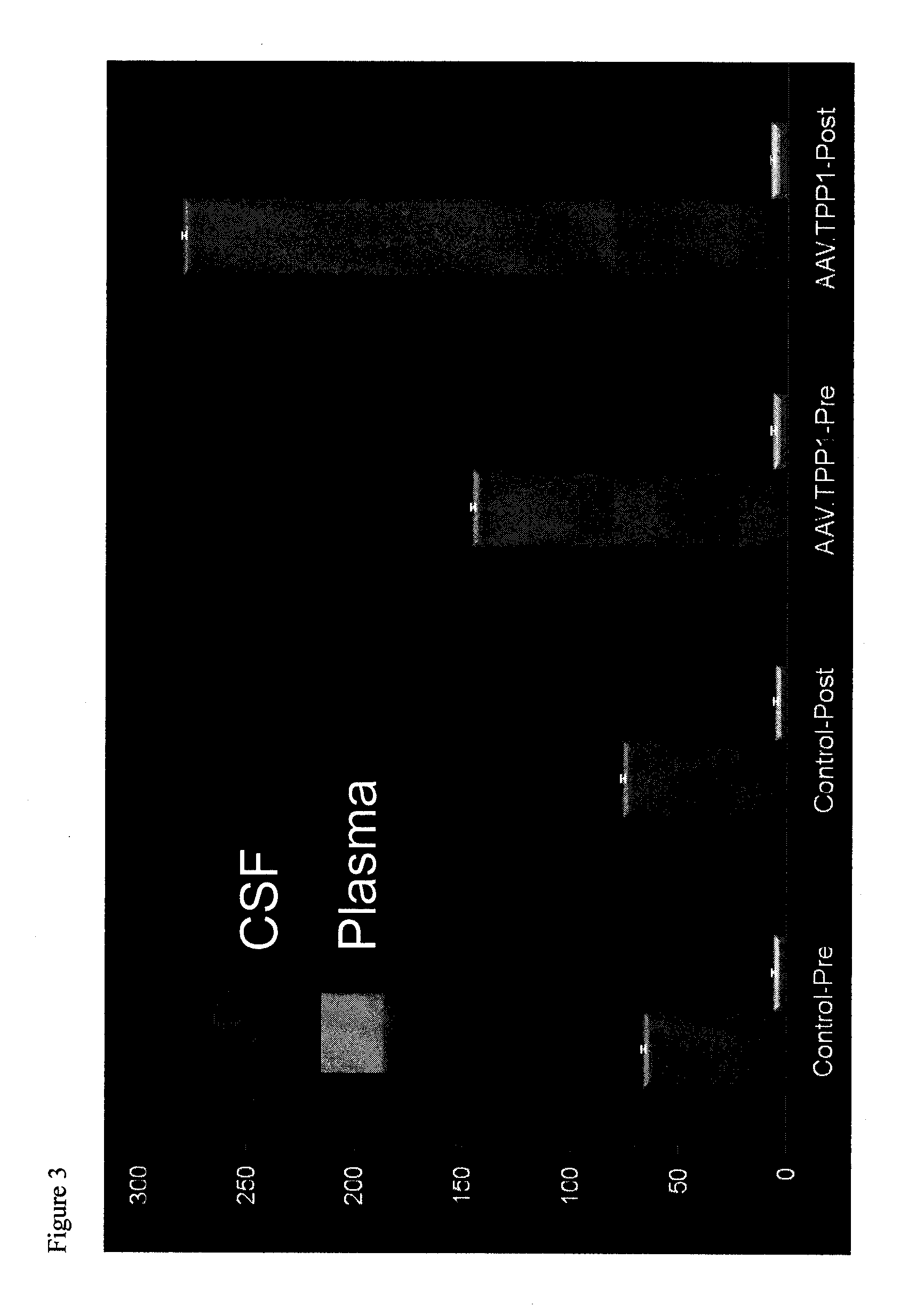
The present disclosure provides methods of treating a disease in a non-rodent mammal comprising administering to the cerebrospinal fluid (CSF) of the non-rodent mammal an rAAV2 particle containing a vector comprising a nucleic acid encoding a therapeutic protein inserted between a pair of AAV inverted terminal repeats in a manner effective to infect an ependymal cell in the non-rodent mammal, wherein the ependymal cell secretes the therapeutic protein so as to treat the disease.
Owner:UNIV OF IOWA RES FOUND
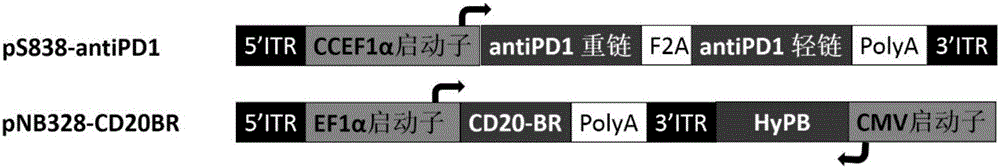
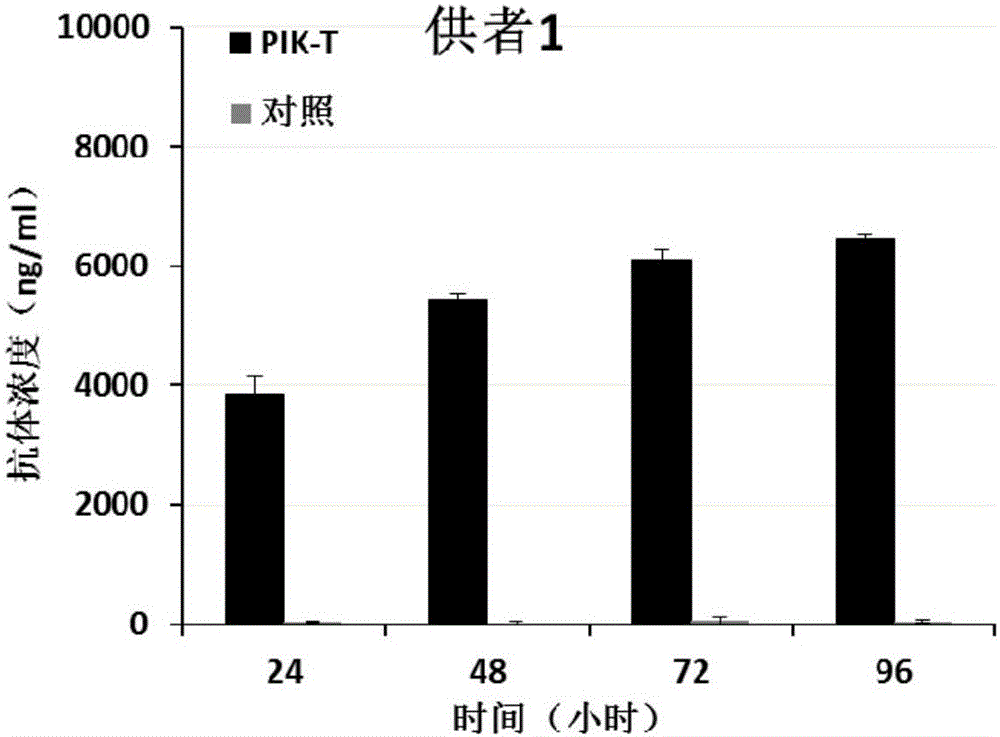
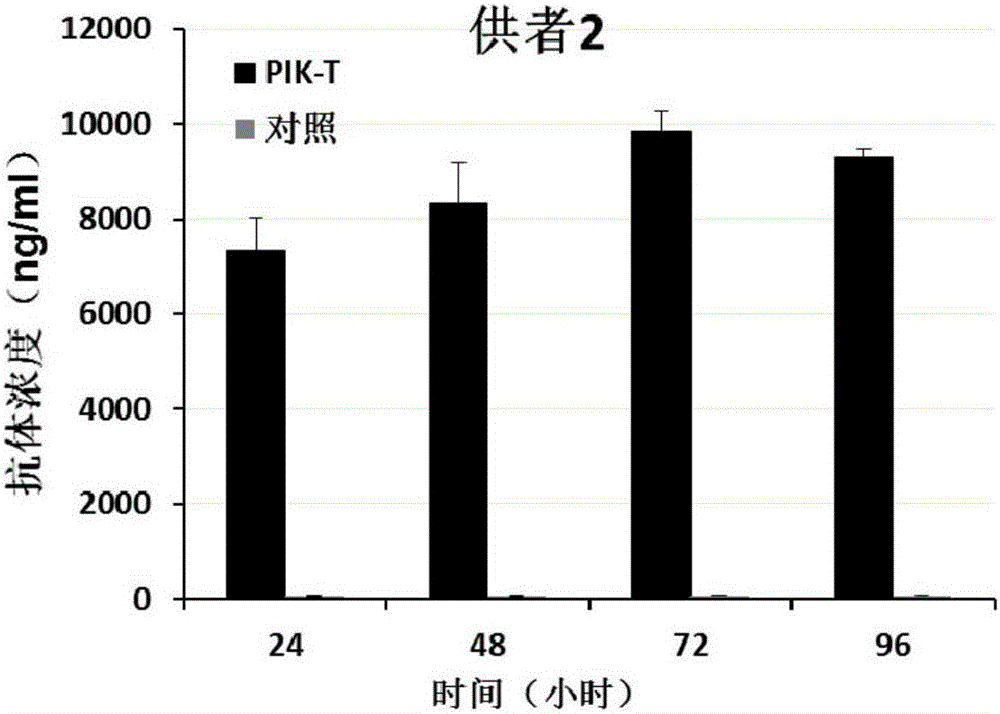
Killing cell for high-efficiency and stable expression of antibodies and use thereof
PendingCN107523545AAntibacterial agentsAntibody mimetics/scaffoldsAntigen Binding FragmentAutoimmune disease
The invention relates to a killing cell for high-efficiency and stable expression of antibodies and a use thereof. Specifically, the invention provides the transgenic killing cell; a genome of the killing cell is stably integrated with an expression cassette containing an encoding sequence of human Fc segment-containing antibodies, or containing an encoding sequence of chimeric antigen receptors and inhibitory antibodies or activated type antibodies, and both ends of the expression cassette contain inverted terminal repeated sequences of a transposon. The killing cell can stably express the human Fc segment-containing antibodies or the chimeric antigen receptors and antigen-binding fragments derived from interested antibodies with high level while maintaining cell killing toxic effect. In addition, for preventing systemic toxicity and autoimmune diseases caused by over expression of antibodies due to in-vivo continuous proliferation of immune cells for stable expression of the antibodies, a molecular braking system is also introduced. With use of monoclonal antibody agents appearing on the market, the killing cell integrated with the antibody expression cassette can be quickly eliminated, and the safety of treatment is effectively improved.
Owner:SHANGHAI CELL THERAPY RES INST +1
Compositions, Methods, and Systems for SIRNA Delivery
InactiveUS20110052666A1Reduce deliveryOrganic active ingredientsNervous disorderPyrimidine NucleotidesBiology
The instant invention provides silencer expression cassettes for delivery of RNAi therapy. The silencer cassettes of the instant invention encode an RNAi agent and lack means for replication within a host cell, inverted terminal repeats and further lacking one or more selectable markers. Optionally, the silencer cassettes lack CpG dinucleotides and / or the RNAi agent is within a scaffold of a naturally occurring miRNA. The composition comprising the silencer cassettes of the instant invention, as well as methods of use and suitable systems are also provided.
Owner:MEDTRONIC INC
Stable Gene Transfer to Proliferating Cells
InactiveUS20170216456A1Stable integrationStable expressionHydrolasesTransferasesDiseaseHepatic Diseases
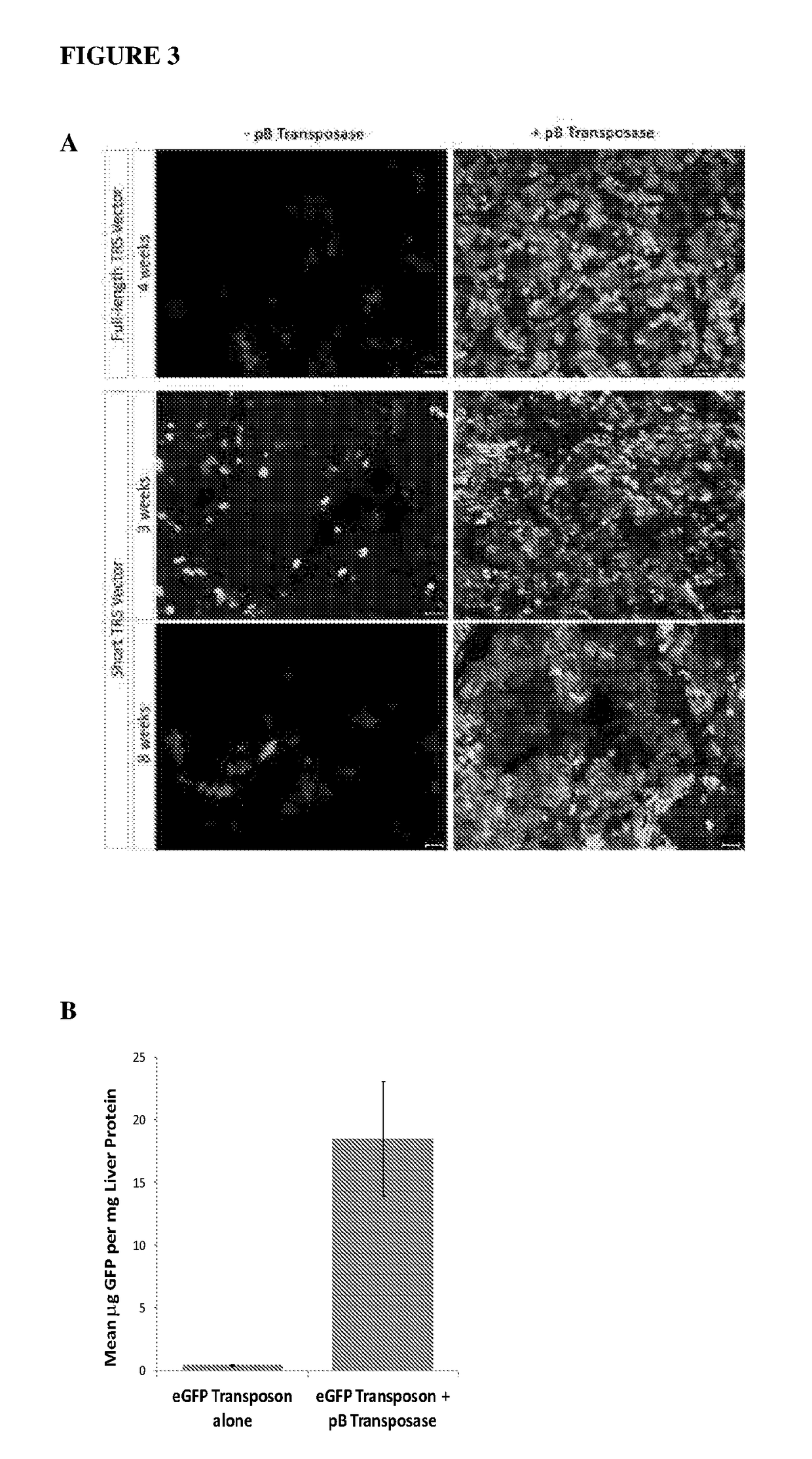
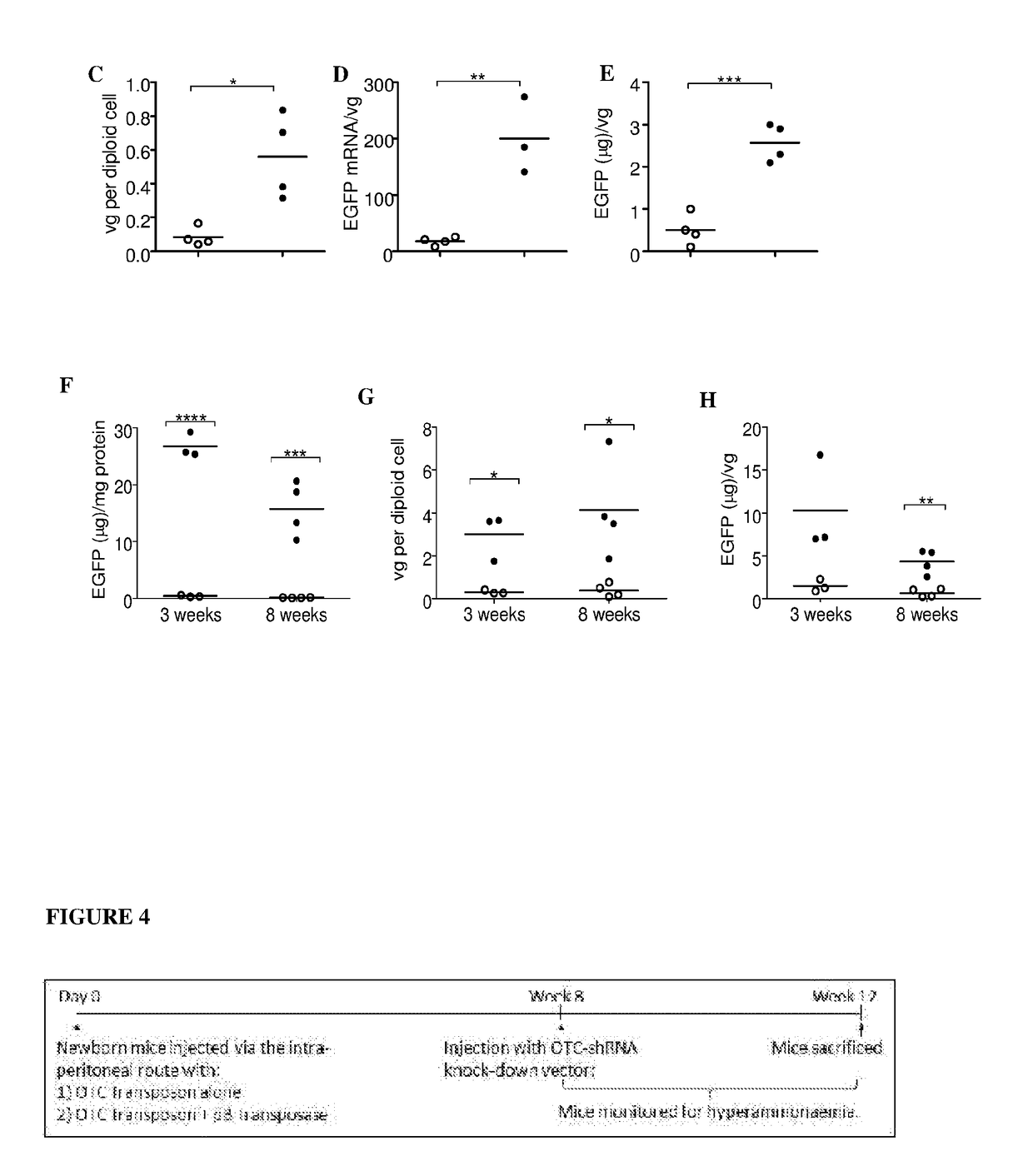
Provided herein are methods for facilitating or inducing stable transgene integration and expression in a proliferating cell, comprising administering to the cell (i) a recombinant AAV (rAAV) vector comprising the transgene flanked by transposon-derived inverted terminal repeat sequences, which sequences are in turn flanked by AAV-derived inverted terminal repeat regions, and (ii) a source of a transposase that recognises said transposon-derived inverted terminal repeat sequences and directs the genomic integration of the transgene into the genome of the proliferating cell. Also provided are methods and transgene delivery systems for the treatment or prevention of diseases affecting, associated with or characterised by proliferating cells, including paediatric liver diseases, bone marrow diseases and cancer.
Owner:MOUNT SINAI HOSPITAL +2
Method for producing heart-specific fluorescence of non-human eukaryotic animals
A method of expressing in vivo heart-specific fluorescence in transgenic line of zebrafish is developed, which provides a research model for studying heart-related gene functions and performing gene therapies in the future. The method comprises the following steps. Firstly, a plasmid is constructed. This plasmid construct includes the upstream regulatory region, the exon 1, the intron 1, and the exon 2 of cmlc2 gene, cDNA of GFP, wherein the cmlc2 gene and GFP cDNA form a cassette, and inverted terminal repeats from adeno-associated virus are flanked at both sides of this cassette. The plasmid construct is linearized and microinjected into one-celled zebrafish fertilized eggs. Lastly, the heart-specific fluorescent expressed zebrafish are selected and the germline-transmitting transgenic strain is generated.
Owner:NAT TAIWAN UNIV
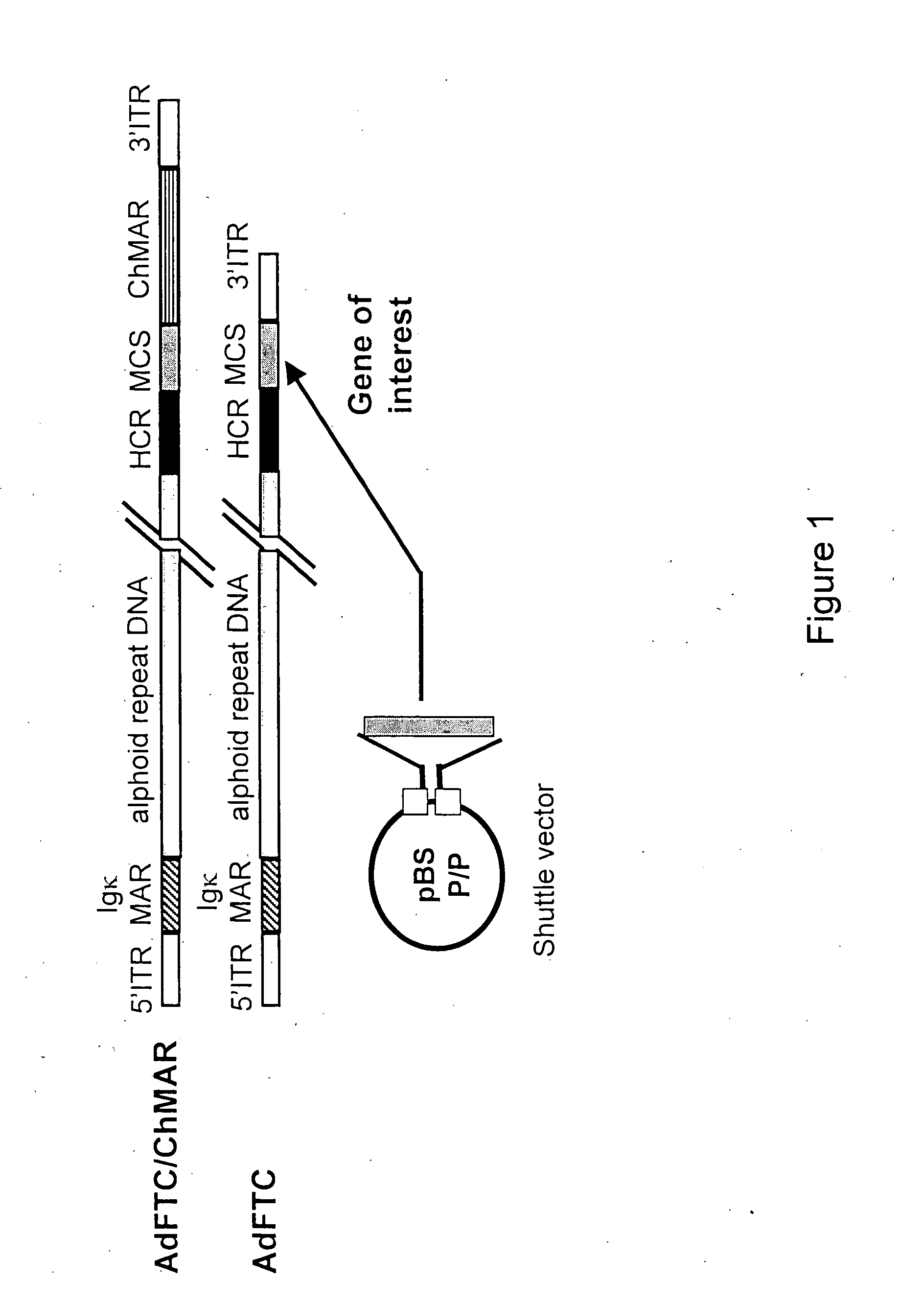
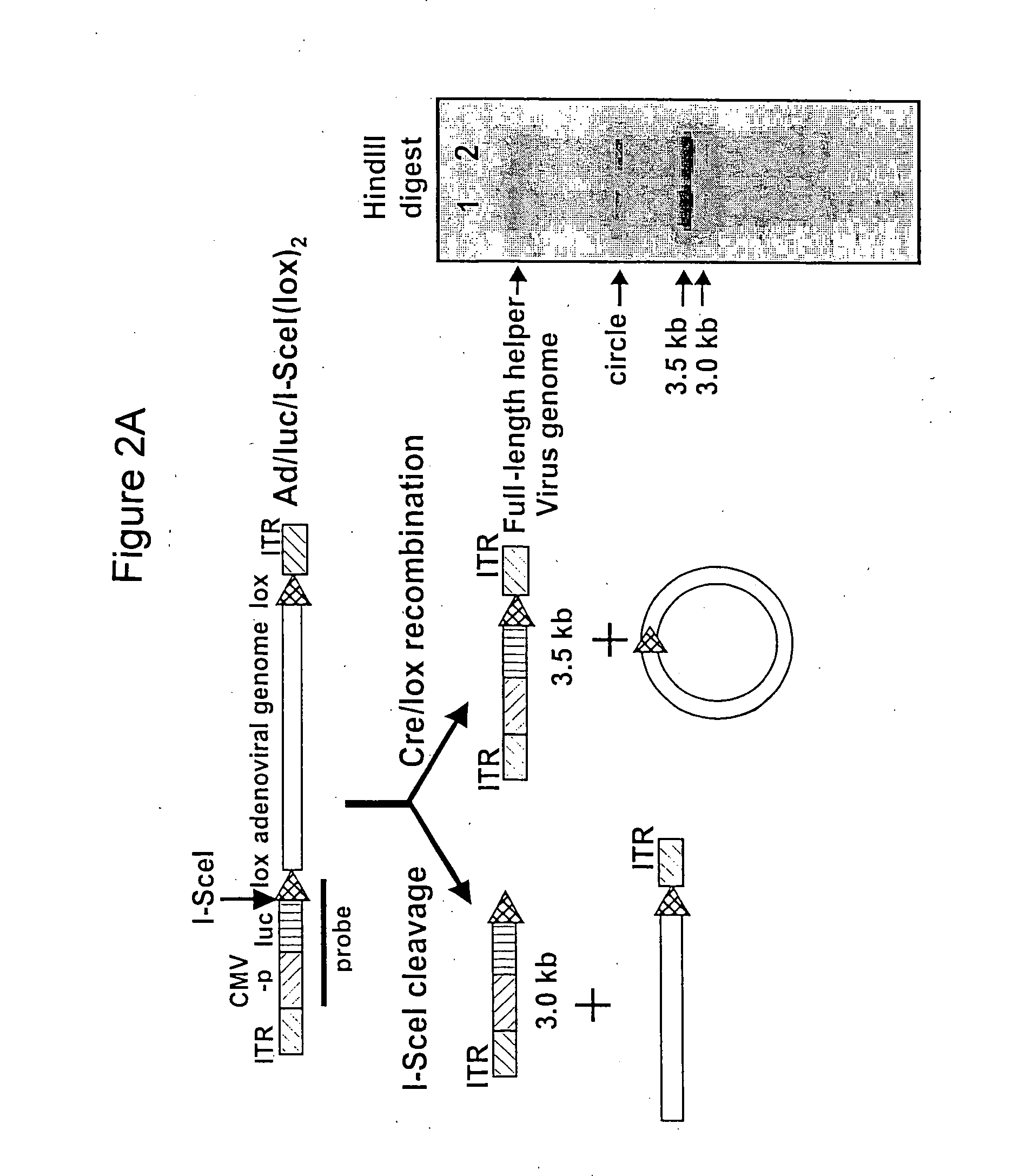
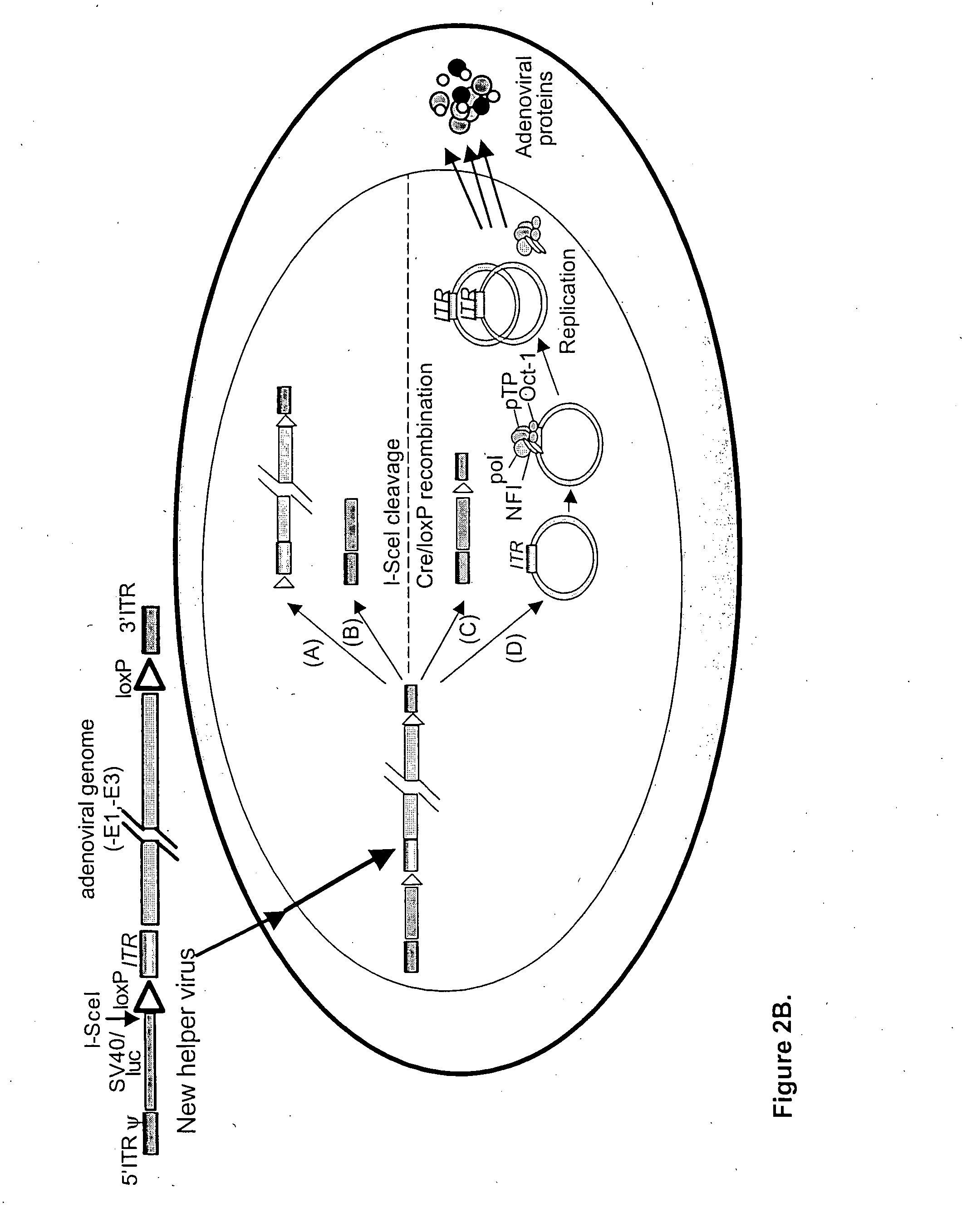
Helper dependent adenoviral vector system and methods for using the same
A helper dependent adenoviral vector system is provided. The subject helper dependent adenoviral vector system is made up of: (1) a "gutless" adenoviral vector, which in certain embodiments includes cis-acting human stuffer DNA that provides for in vivo long term, high level expression of a coding sequence present on the vector, where in certain embodiments the vector includes an integrating domain; (2) an adenoviral helper vector that is characterized by having an adenoviral genome region flanked by recombinase recognition sites, where the helper vectors further include a non-mammalian endonuclease recognition site positioned outside of the adenoviral genome region and in certain embodiments a third adenoviral inverted terminal repeat (ITR) sequence positioned between first and second terminal ITRs; and (3) a mammalian cell that expresses the corresponding recombinase and endonuclease, as well as the adenoviral preterminal and polymerase proteins. Also provided are methods of using the subject systems to produce virions having the subject helper dependent adenoviral vectors encapsulated in an adenoviral capsid. In addition, kits for use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com