Constructs and Methods for Delivering Molecules via Viral Vectors with Blunted Innate Immune Responses
a technology of innate immune responses and constructs, which is applied in the direction of viruses/bacteriophages, biocide, genetic material ingredients, etc., can solve the problems of recombinant aav, attendant immune responses that have compromised the outcome of aav-mediated gene therapy, and the inability to integrate into the genome, so as to improve adeno-associated virus (aav)-mediated gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
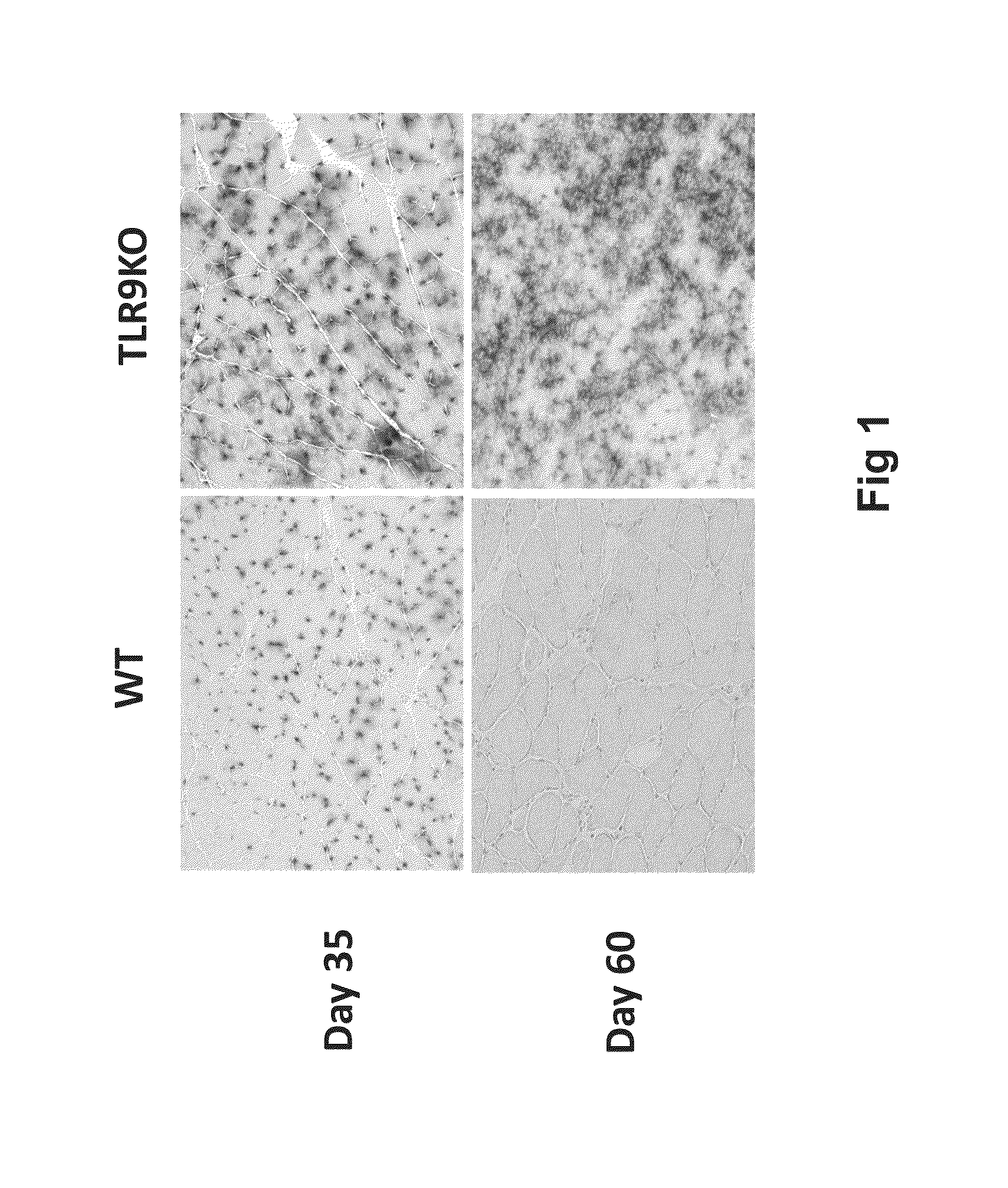
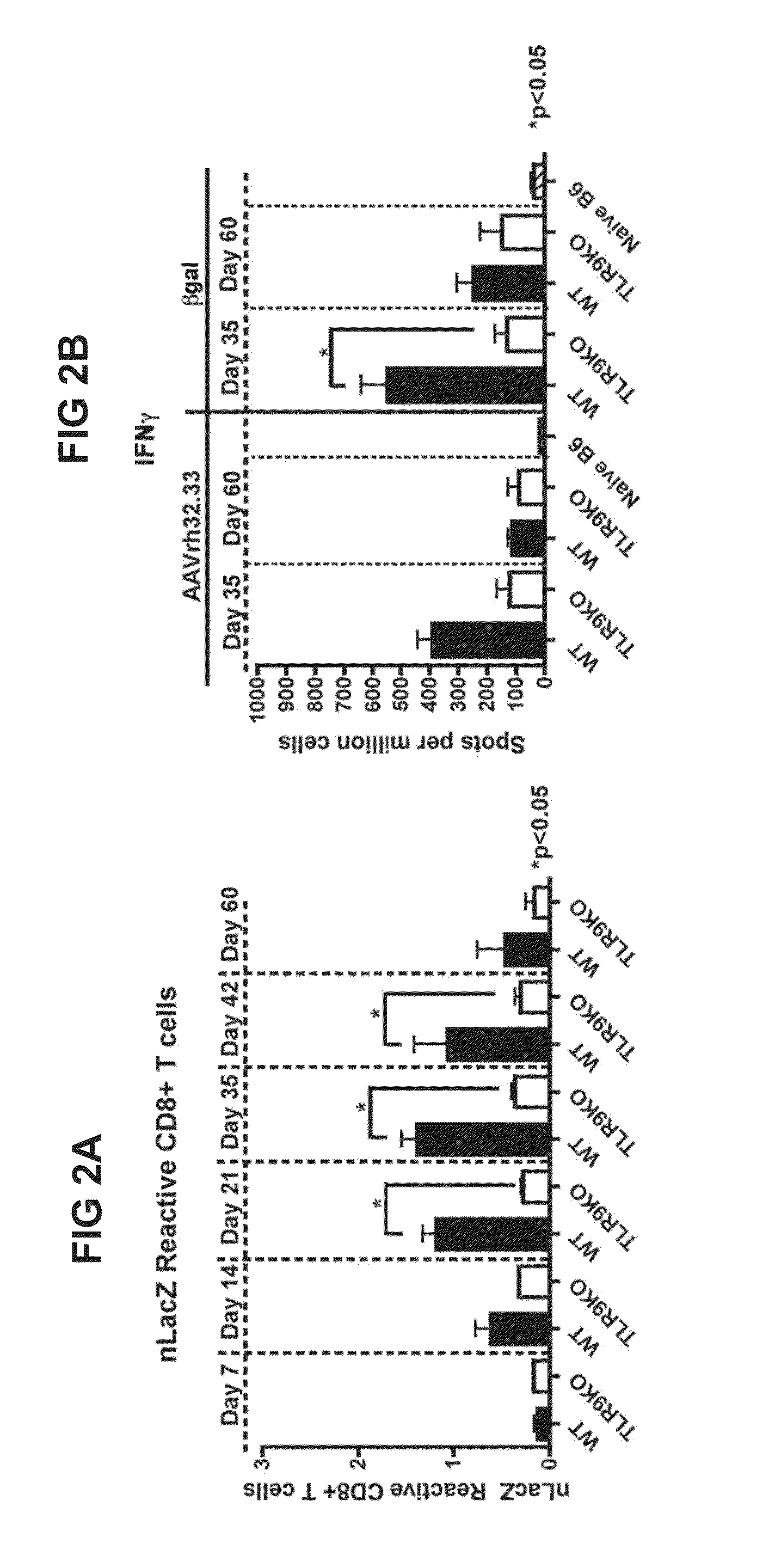
In the Absence of TLR9 Signaling, AAVrh32.33nLacZ Muscle Gene Transfer Results in Stable Transgene Expression
[0135]The current study assessed the requirement for TLR9 signaling in T cell immunoreactivity and transgene loss in response to AAVrh32.33. These mechanistic findings were subsequently translated into a modified, CpG-depleted AAVrh32.33 vector that escapes the adaptive immune response and exhibits stable, long-term transgene expression.
[0136]A. Material and Methods:
[0137]1. Mice: C57BL / 6 wild type (WT) mice were ordered from The Jackson Laboratory. Toll-like receptor 9 knockout (TLR9KO) mice were a kind gift from Dr. Phillip Scott (University of Pennsylvania, Philadelphia, Pa.). All mice were housed under specific pathogen-free conditions in the TRL Animal Facility at the University of Pennsylvania. All animal procedure protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
[0138]2. Adeno-Associated Viral Vectors (AAV) me...
example 2
[0177]To measure LacZ expression of RhCpG+ and CpG− constructs, HeLa cells were transfected with CpG+ and CpG− AAV expression plasmids. Four days post transfection cells were assayed for β-galactosidase activity using the Mammalian β-galactosidase assay kit as instructed for adherent cells. Absorbance was measured at 405 nm on a TECAN Infinite M1000 PRO plate reader. CpG+ and CpG− AAV vector constructs exhibited comparable LacZ plasmid expression. These data demonstrate that transgene loss in the skeletal muscle of RhCpG+ gene transferred is not due to differential β-gal expression levels at the plasmid level.
[0178]To assess transgene stability and cellular infiltrate at an early kinetic time point, mice were injected intramuscularly with 1×1011 GC of RhCpG+ or RhCpG− vector. 14 days post vector injection, gastrocnemius was harvested and skeletal muscle cryosections were stained with CD4 or CD8 monoclonal antibody (MAb) as well as X-gal. Stable transgene expression and minimal cellu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| kinetic time | aaaaa | aaaaa |
| kinetic time | aaaaa | aaaaa |
| kinetic time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com