Stable Gene Transfer to Proliferating Cells
a technology of proliferating cells and gene transfer, applied in the direction of transferases, ligases, genetic material ingredients, etc., can solve the problems of hammering the further development of aav-based gene therapy approaches, and achieve the effect of stable integration and expression of transgenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
posase Vector Constructs
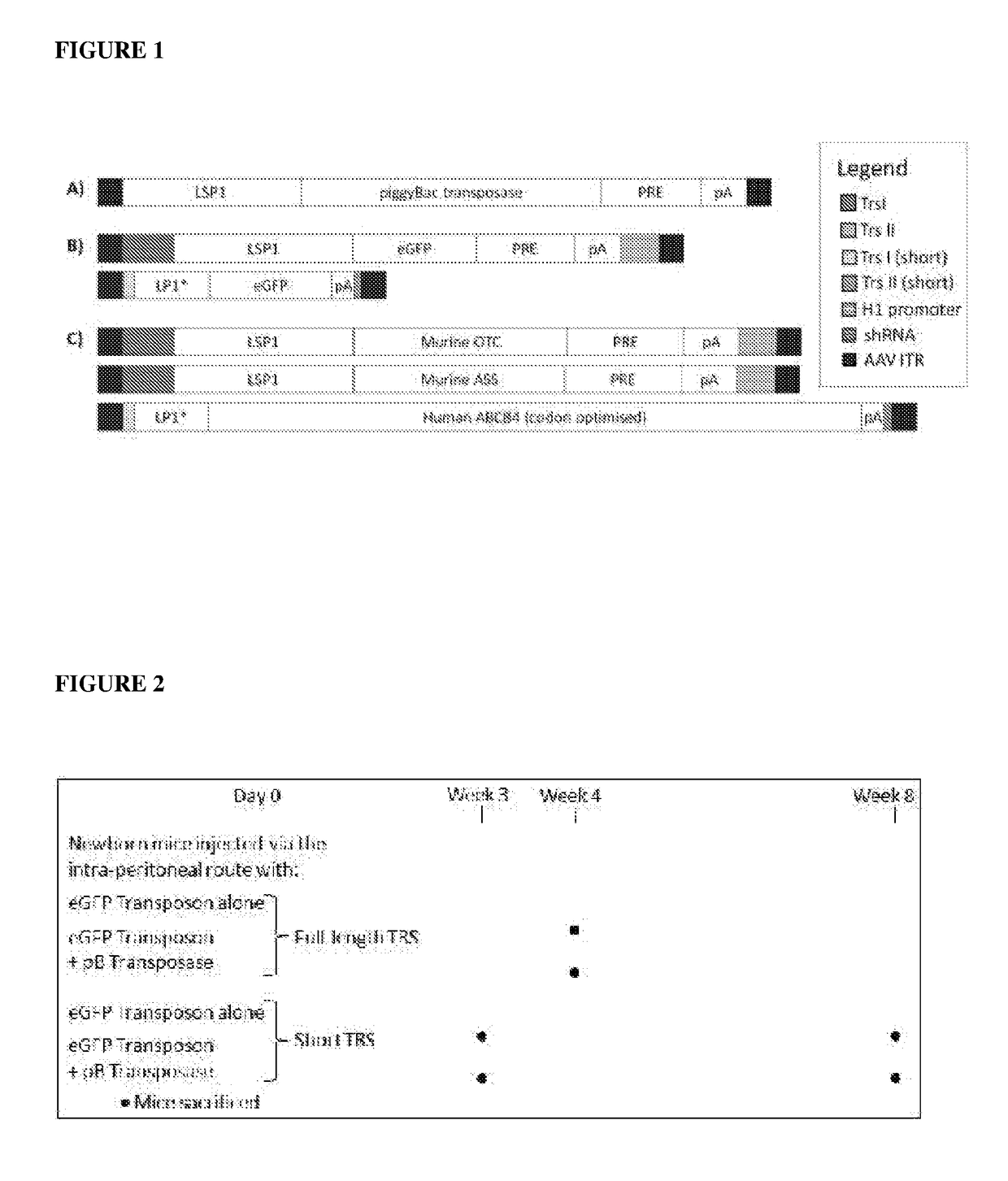
[0070]Transposon-donor vectors and a piggyBac Transposase vector were constructed using the recombinant adeno-associated viral vector (rAAV) system. The hybrid AAV / transposase system was subsequently used (see Examples 2 to 4) to demonstrate phenotype correction in animal models with genetic metabolic disease phenotypes. These included the spfash mouse model of ornithine transcarbamylase (OTC) deficiency and the citrullinaemic mouse model of argininosuccinate synthetase (ASS) deficiency (both urea cycle disorders), and the PFIC3 mouse model (ABCB4 deficiency) of progressive familial intrahepatic cholestasis. Each of these disease phenotypes presents early in life, in neonates or juveniles.
[0071]The coding sequence of piggyBac transposase was amplified by PCR from pCAG-PBase. The piggyBac transposase vector was constructed by inserting the coding region of the piggyBac transposase into a rAAV2 genome under the transcriptional control of a liver-specific promot...
example 2
pression of Hybrid AAV / Transposase Constructs in Mice
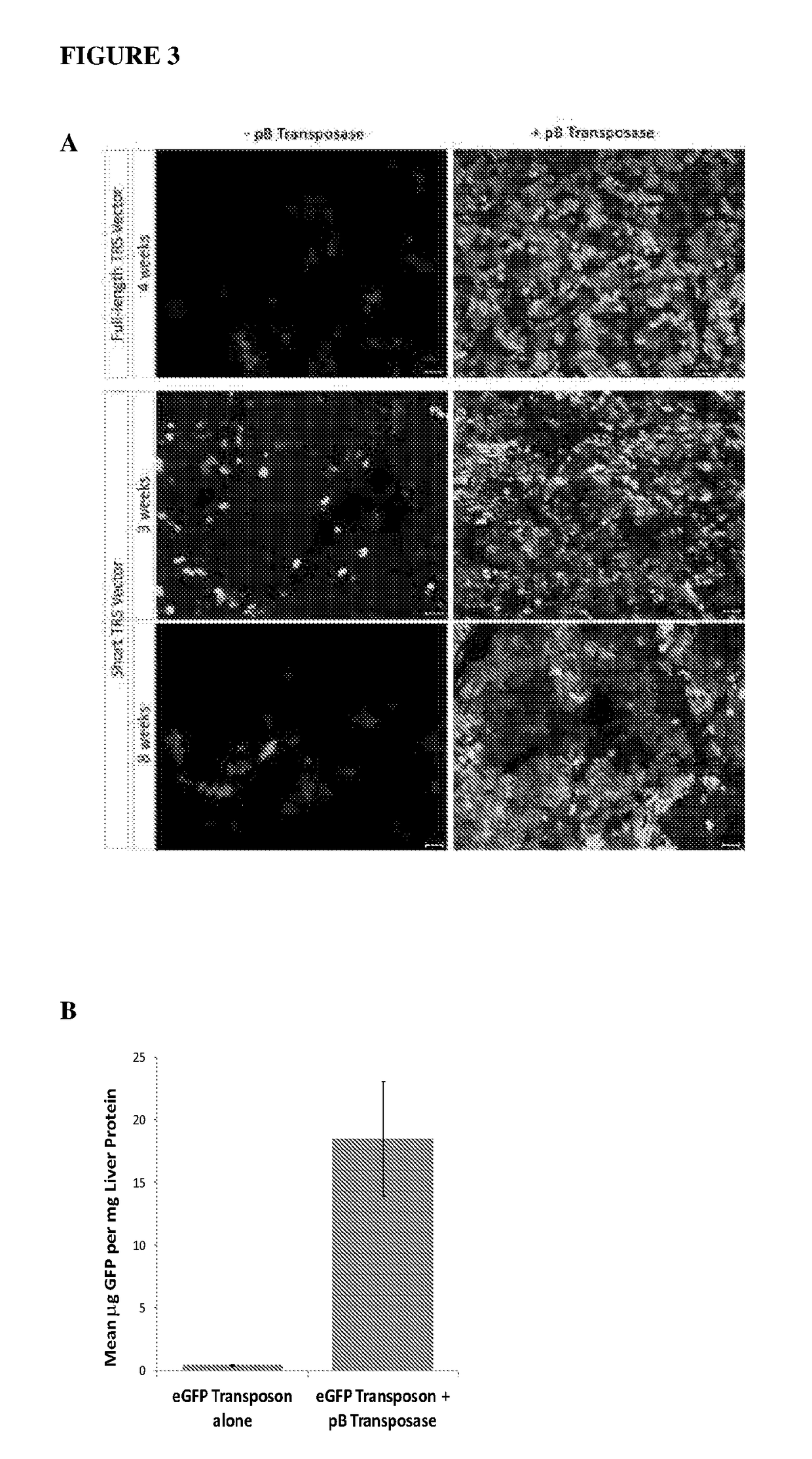
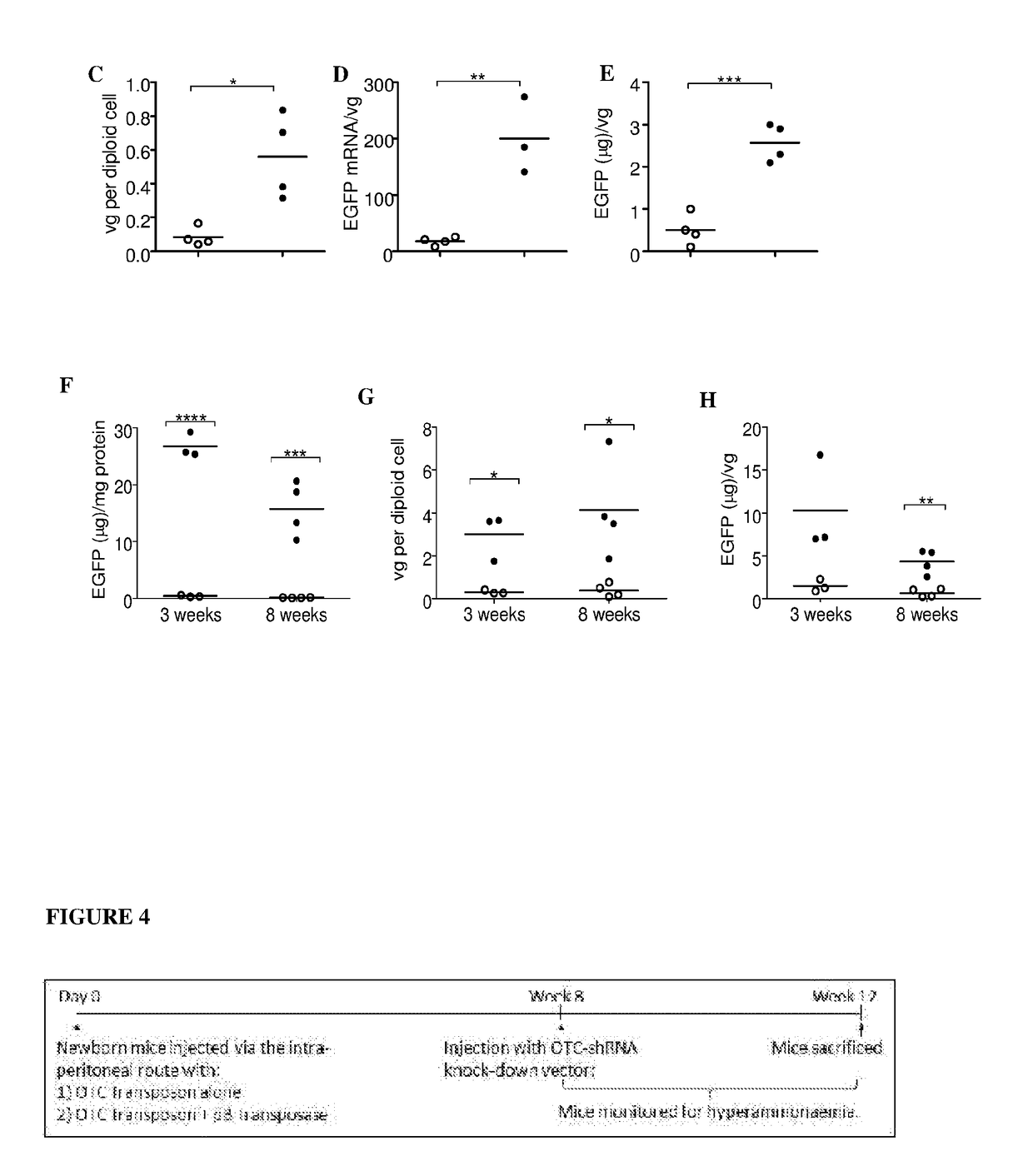
[0131]The ability of the AAV / transposase vector systems described in Example 1 to stably integrate and express a transgene in a host genome was determined using transposon-donor vectors encoding enhanced green fluorescent protein (EGFP) administered to C3H and FVB.129P2-Abcb4tm1Bor mice. Animals were housed in a temperature-controlled environment with 12-hour light / dark cycles with water and standard rodent chow (18.9% (wt / wt) protein; Specialty Feeds, Glen Forrest, Australia) supplied ad libitum. All experimental procedures were evaluated and approved by the institutional Animal Care and Ethics Committee. The experimental design is outlined in FIG. 2. Four mice were used for each group. Constructs were administered by injection via the intraperitoneal route in 20 μL volumes (diluted in PBS with calcium and magnesium) in newborn mice, at vector doses of 5×1010 vg / mouse for the transposase vector, and 1×1011-5×1011 vg / mouse for the...
example 3
apy in a Mouse Model of OTC Deficiency
[0138]The hybrid AAV / transposase constructs described in Example 1 were used to demonstrate phenotype correction in a mouse model of OTC deficiency (the spfash mouse model). Mice used were strain B6EiC3Sn a / A-Otcspf-ash / J (provided by The Jackson Laboratory). The disease phenotype presents early in life in neonates or juveniles. As such, vector treatment was delivered to mice during the neonatal period. Constructs were administered by injection via the intraperitoneal route in 20 μL volumes (diluted in PBS with calcium and magnesium) in newborn mice (1-2 days), at vector doses of 5×1010 vg / mouse for the transposase vector, and 1×1011 vg / mouse for the transposon-transgene donor vector. The experimental design is outlined in FIG. 4 with 12 mice receiving OTC-transposon-encoding AAV2 / 8 vector alone, and 12 mice receiving OTC transposon-encoding vector in combination with the piggyBac transposase-encoding AAV2 / 8 vector.
[0139]Liver sections from mice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com