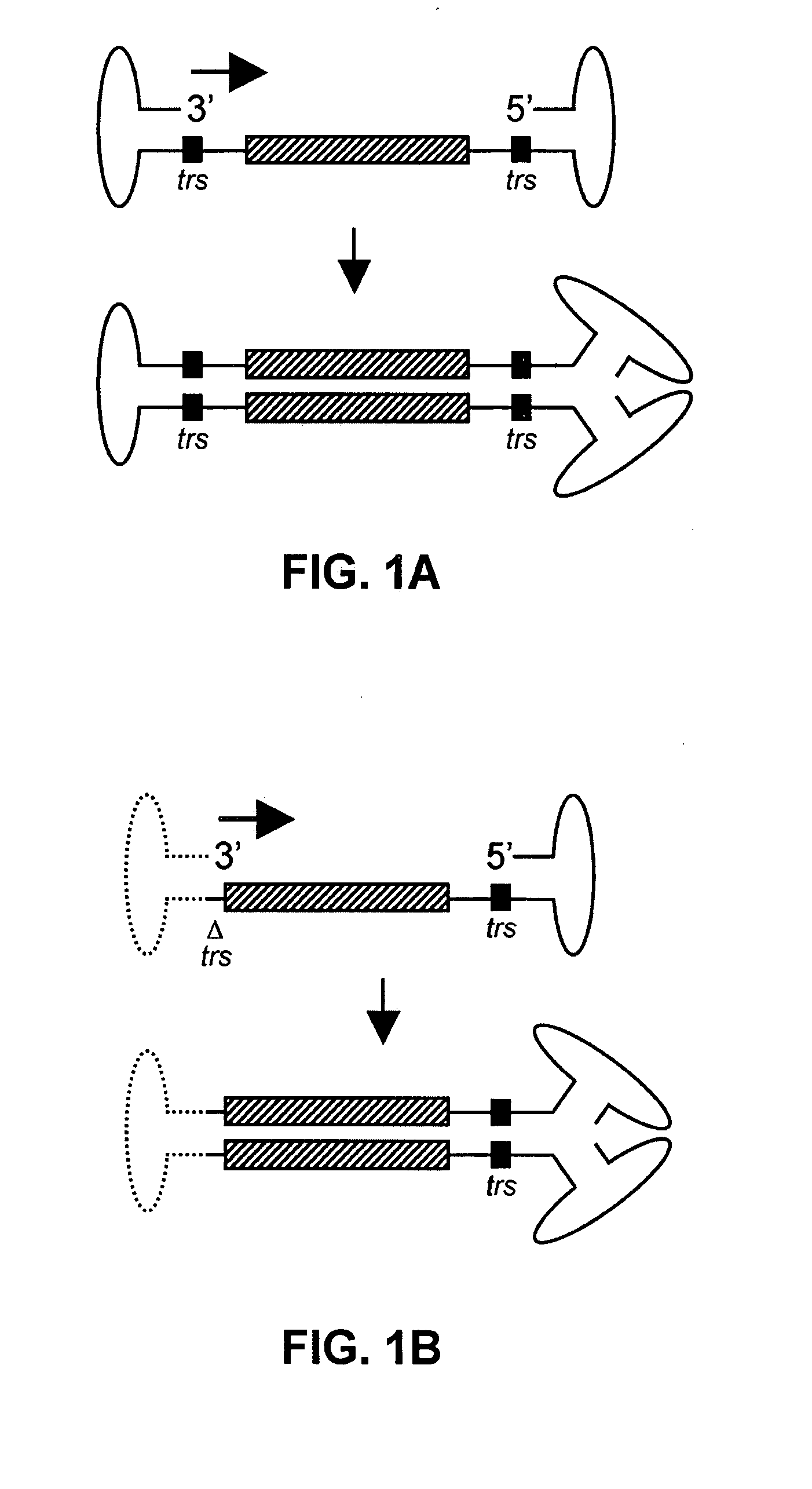
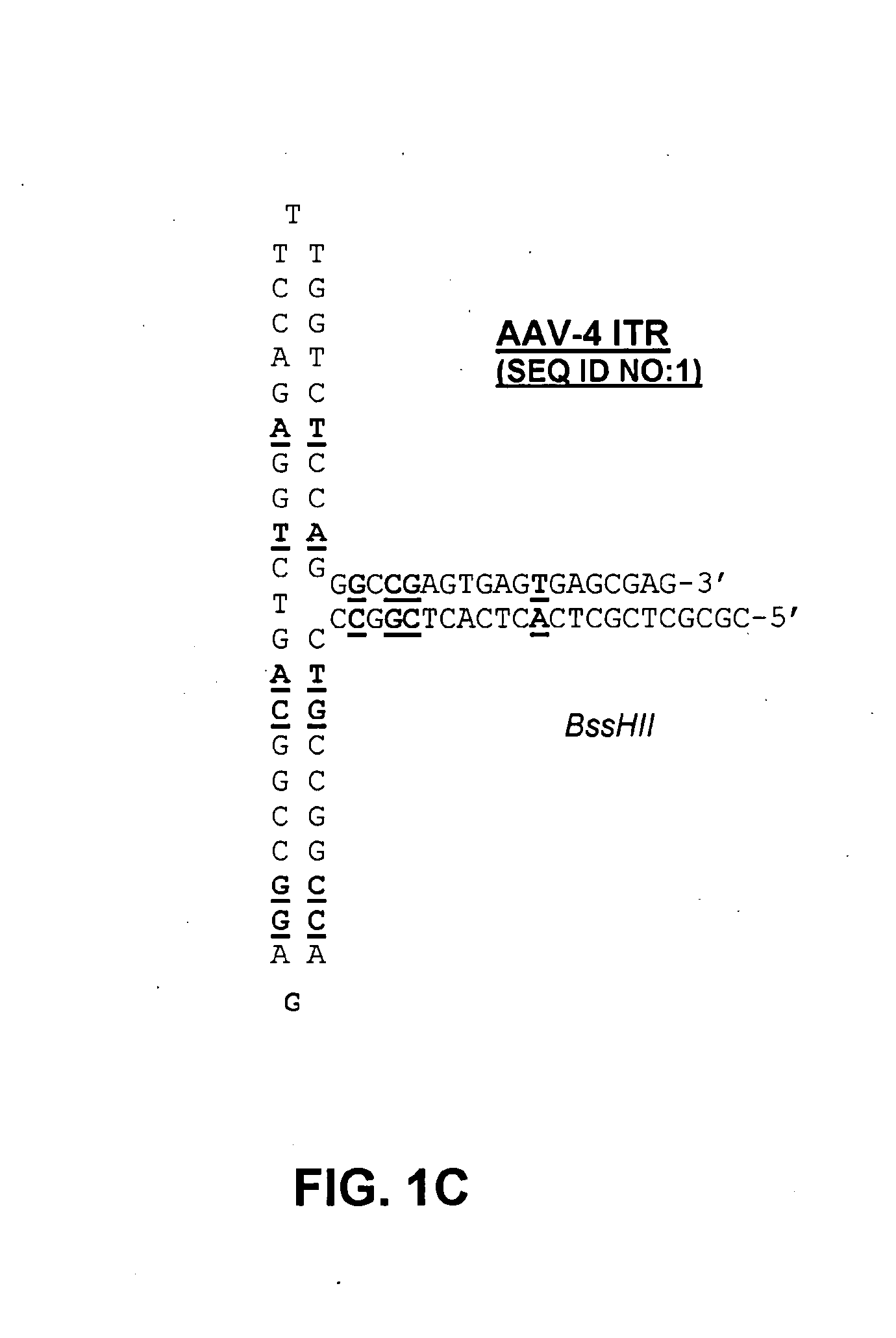
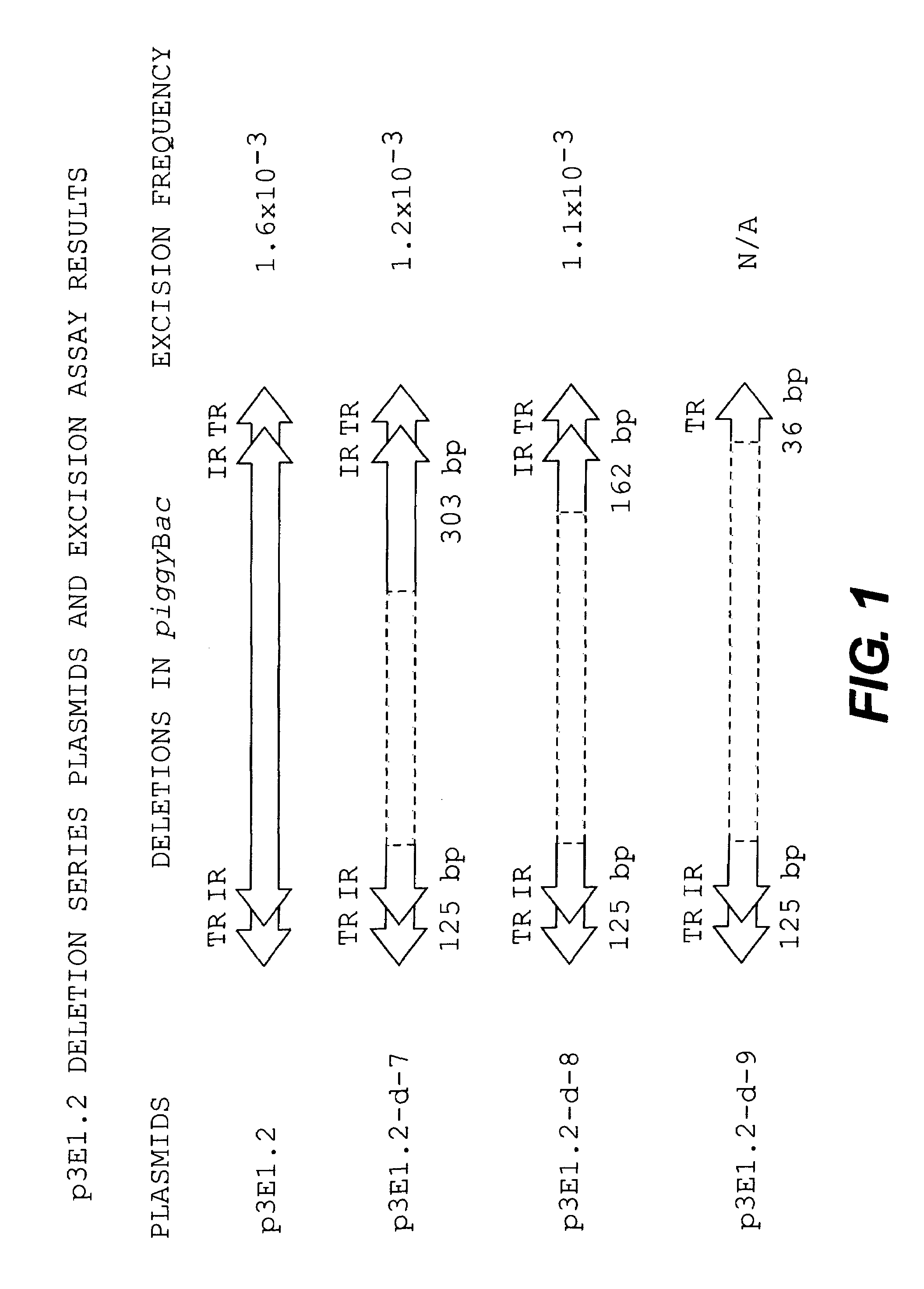
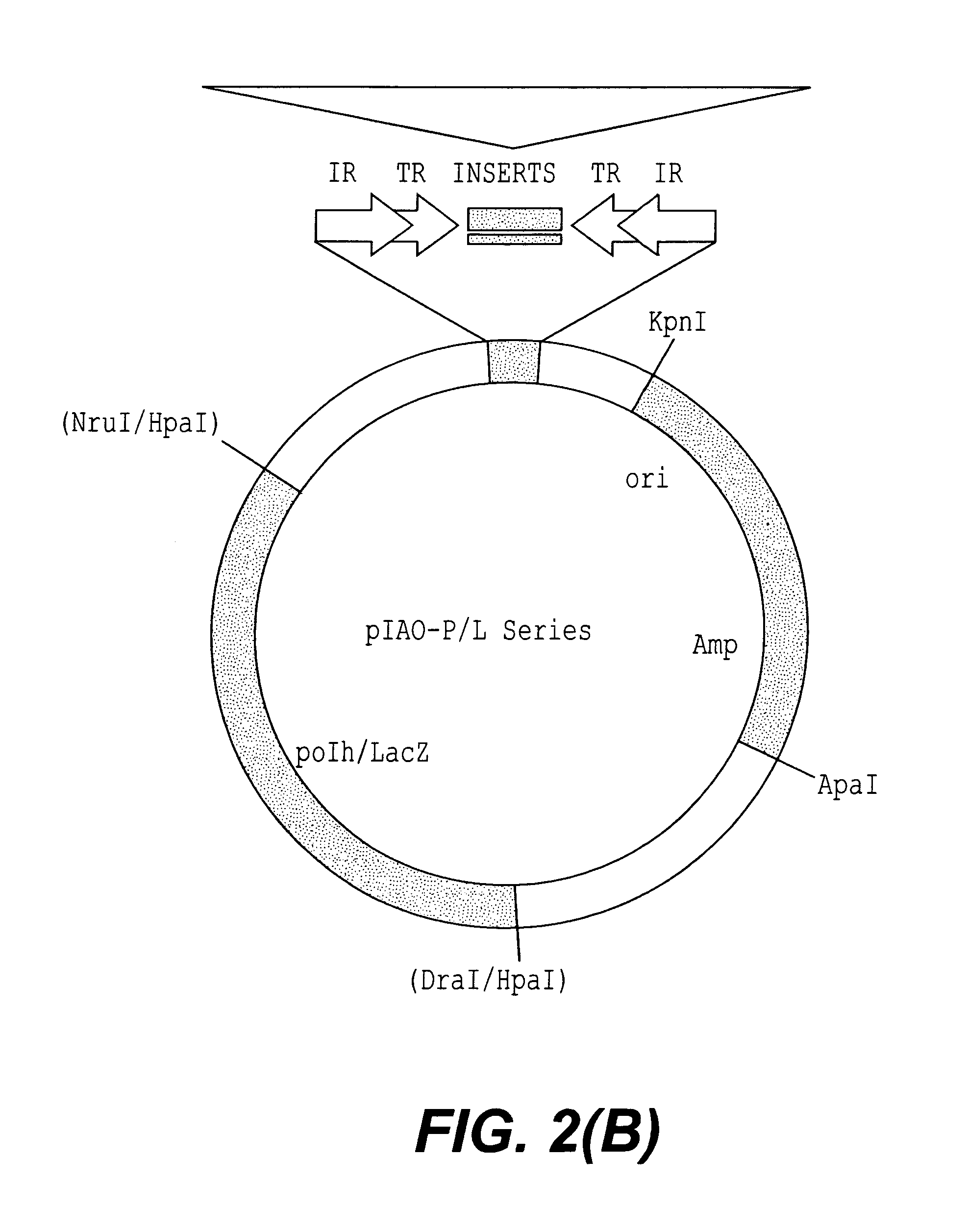
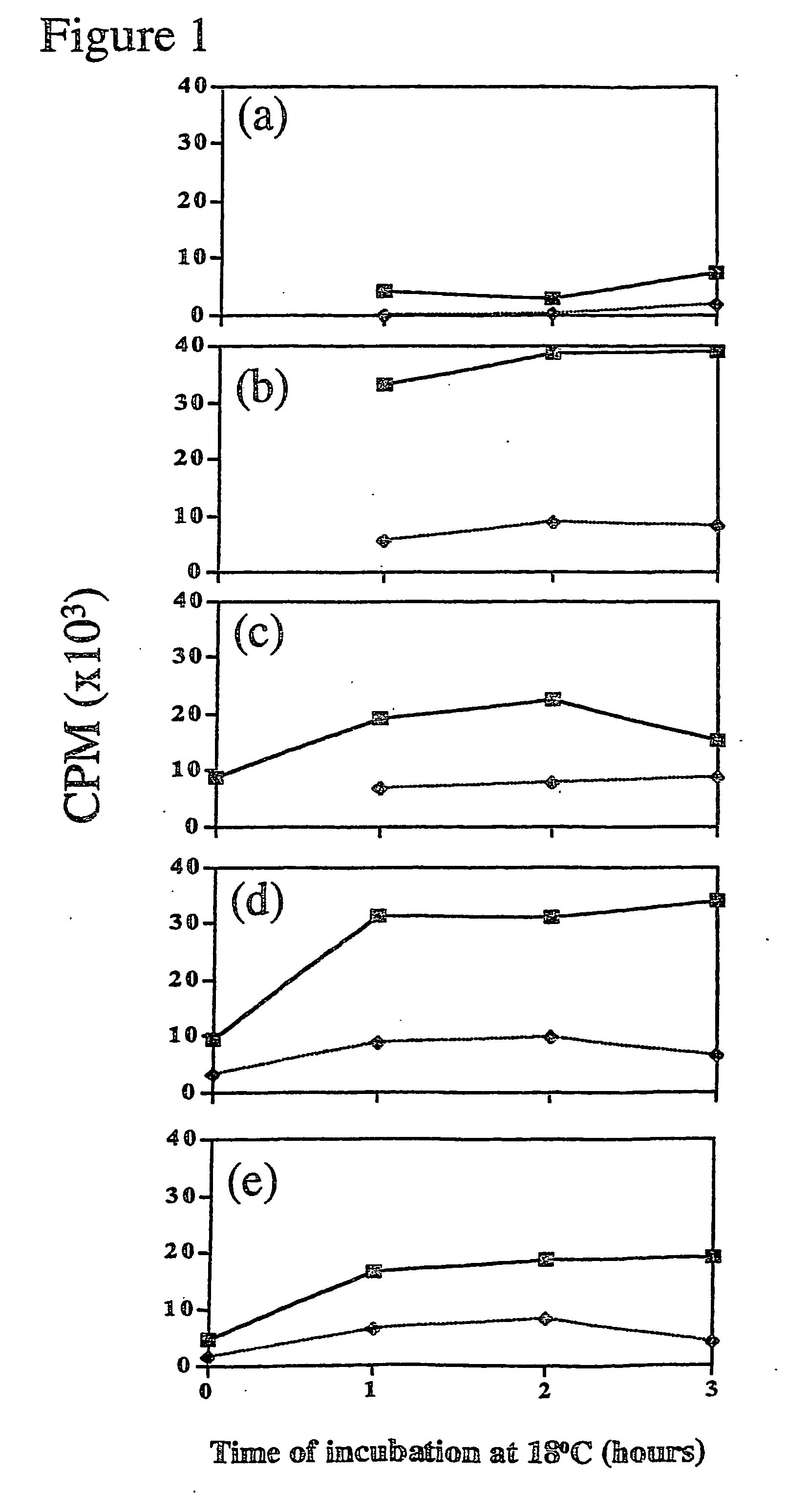
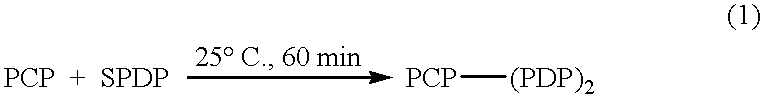
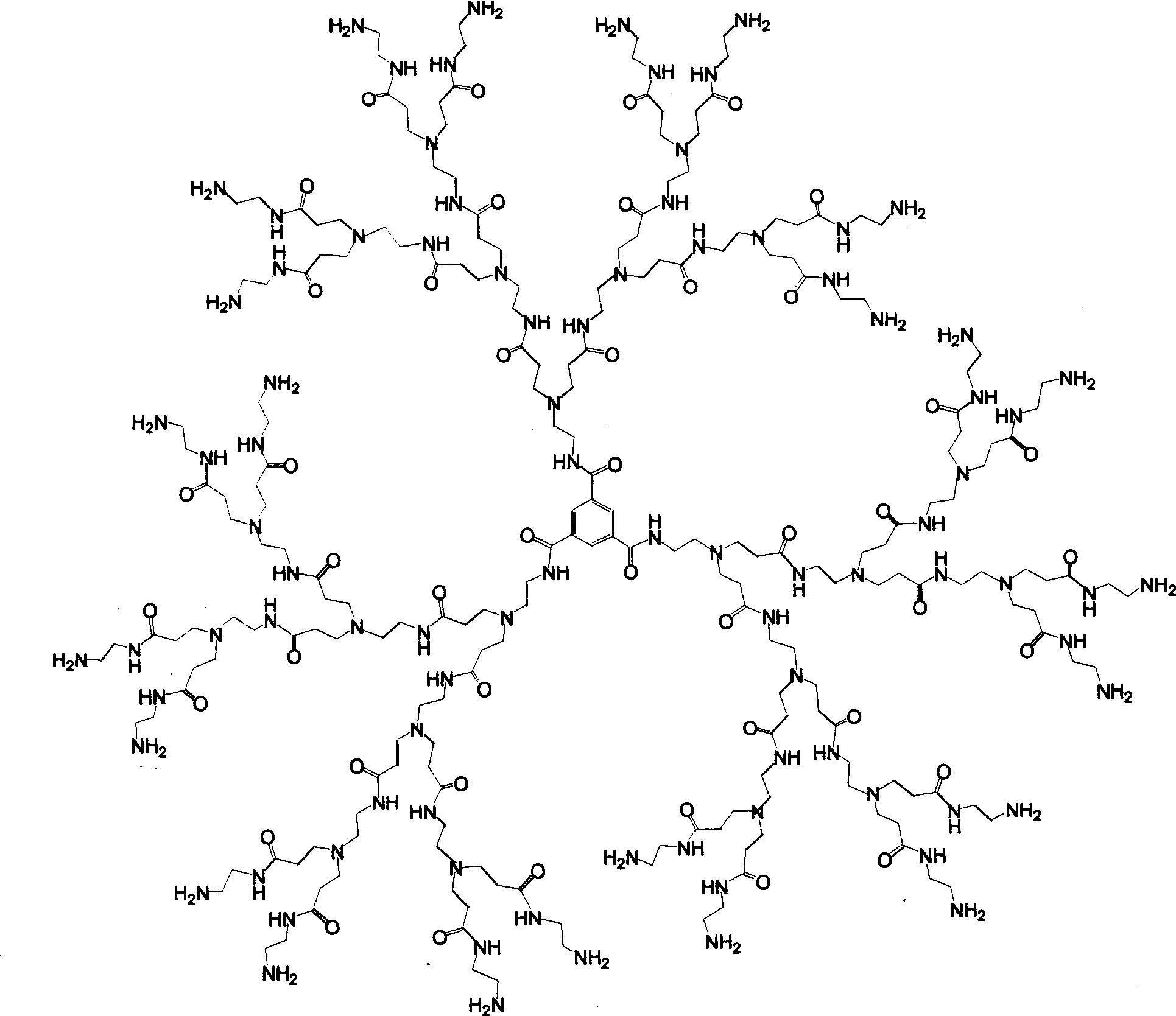
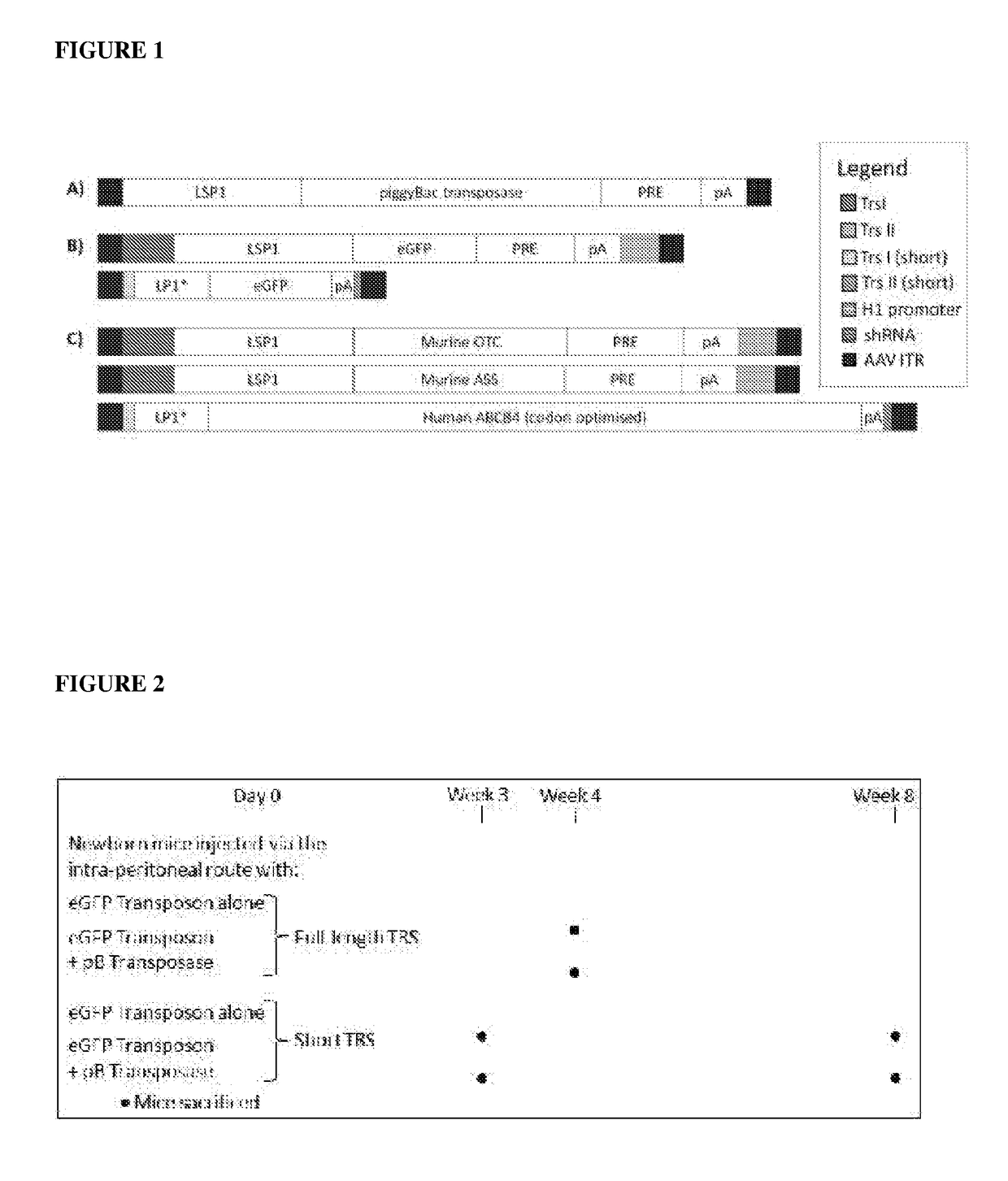
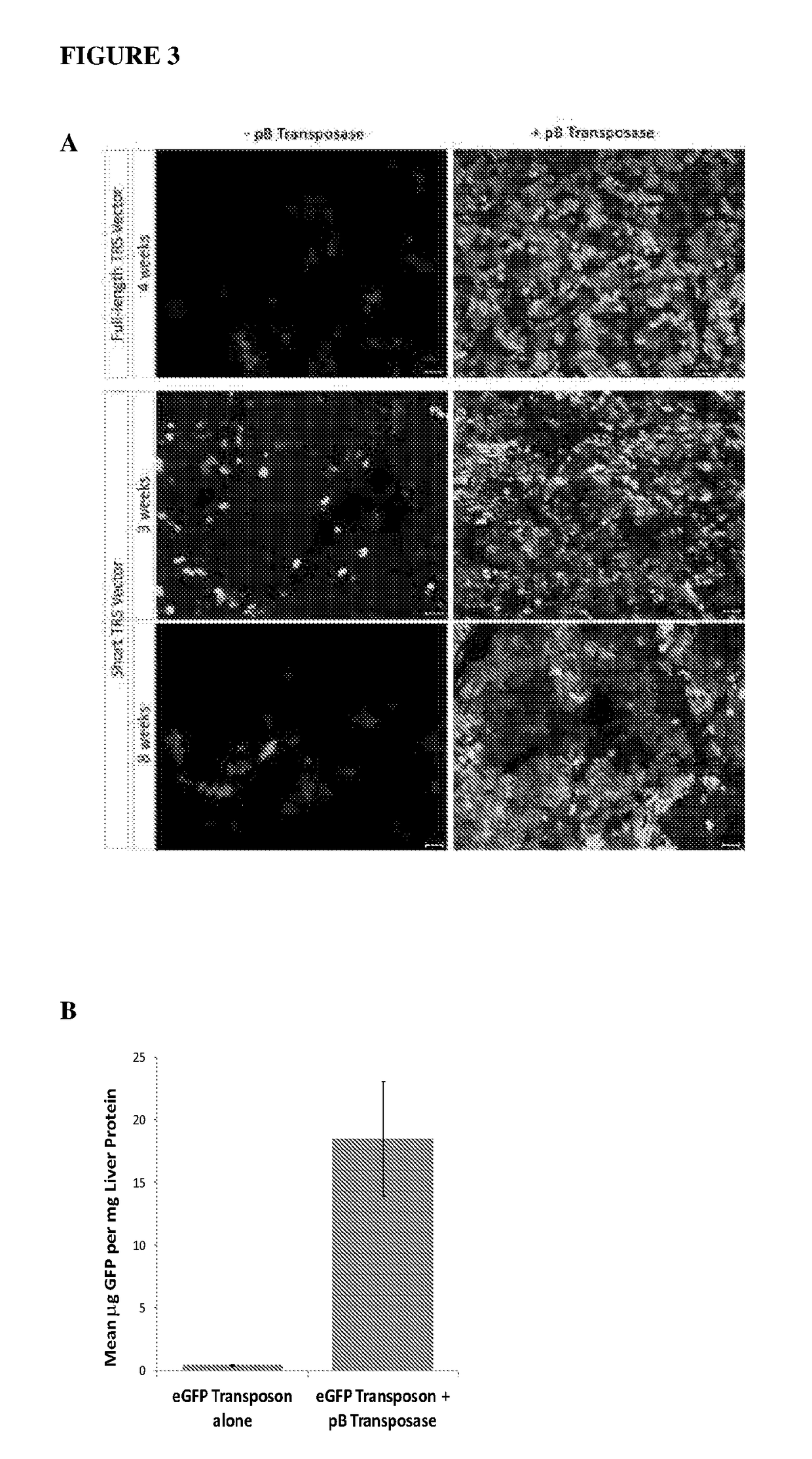
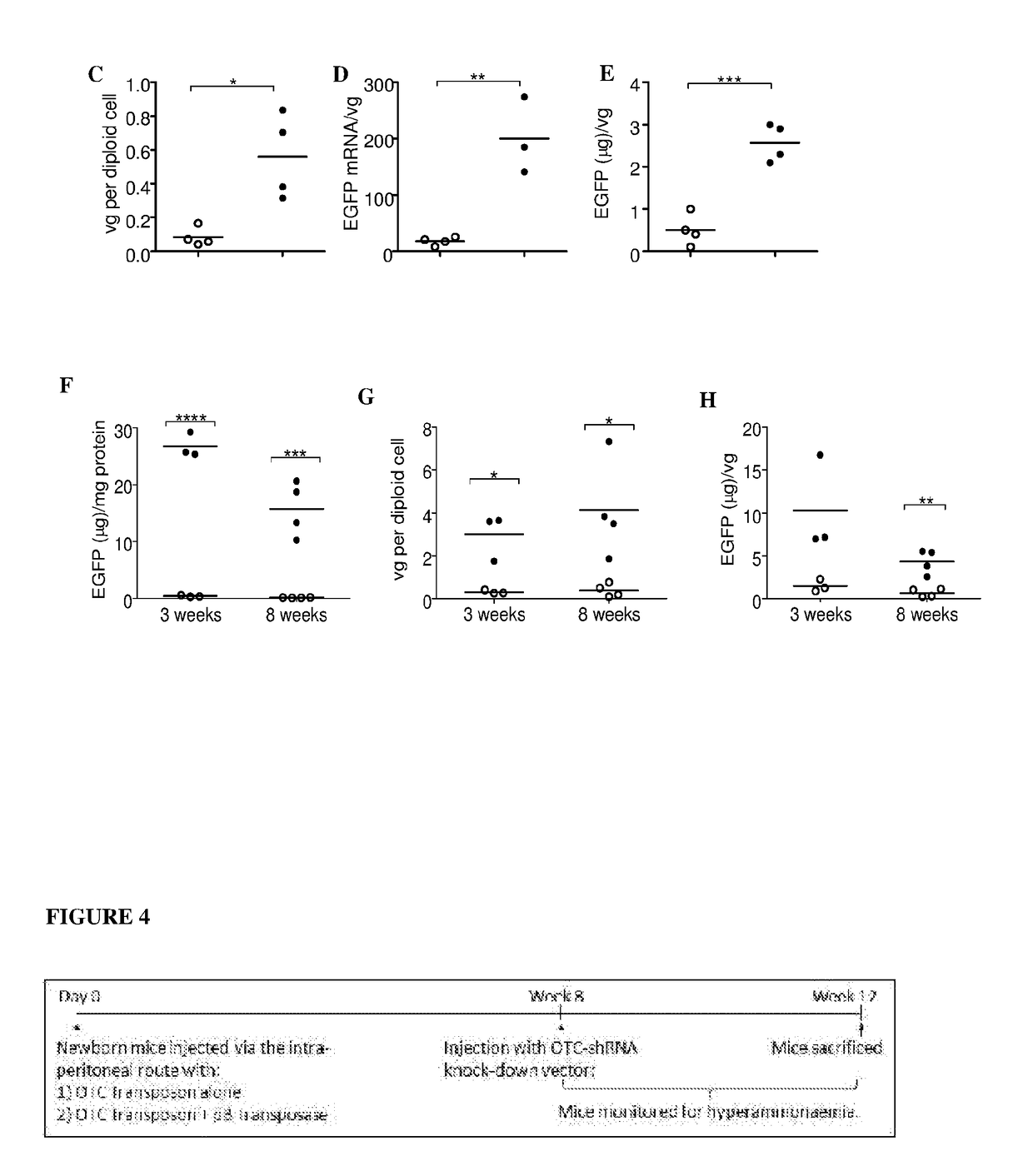
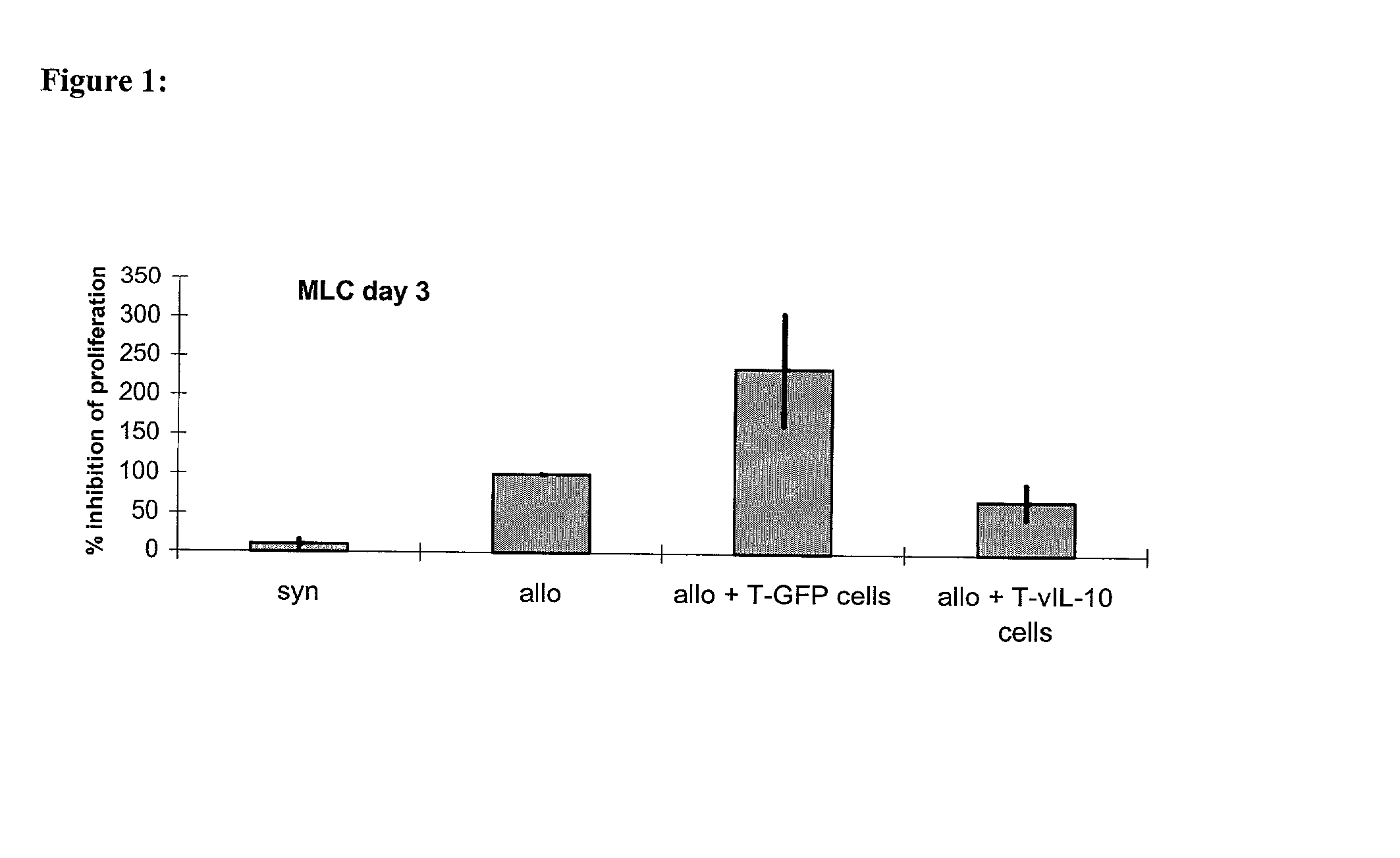
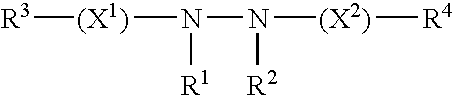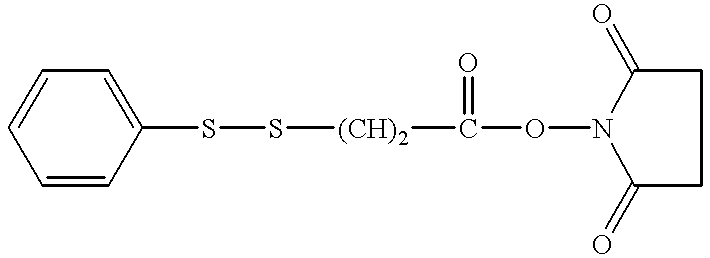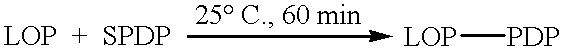Patents
Literature
261 results about "Genetic transfer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
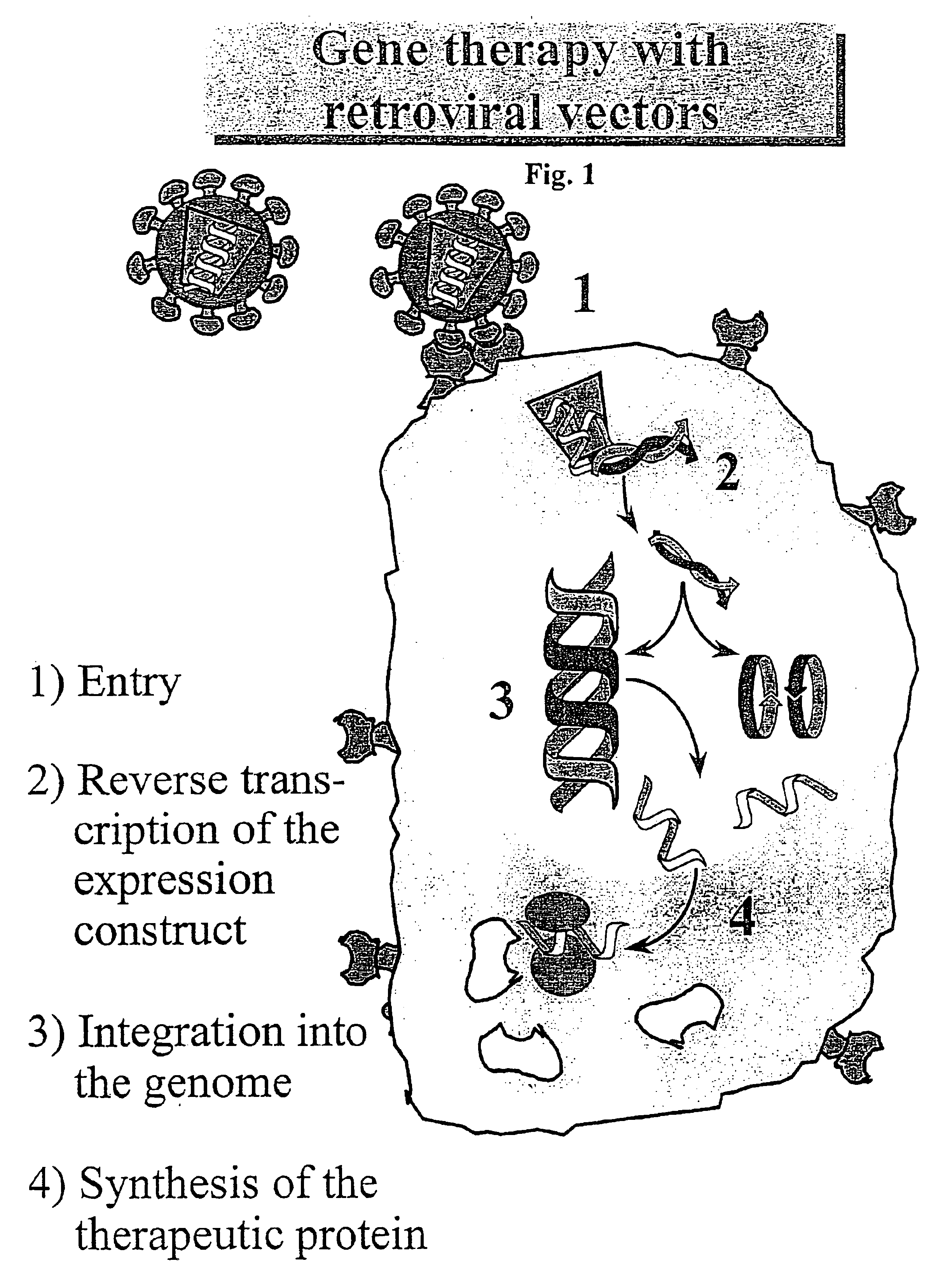
Gene transfer: The insertion of unrelated genetic information in the form of DNA into cells. There are different reasons to do gene transfer. Perhaps foremost among these reasons is the treatment of diseases using gene transfer to supply patients with therapeutic genes.
Adeno-Associated Virus Virion for Gene Transfer to Nervous System Cells
ActiveUS20130224836A1Improve efficiencyLower Level RequirementsNervous disorderSpecial deliveryNervous systemAdeno associate virus
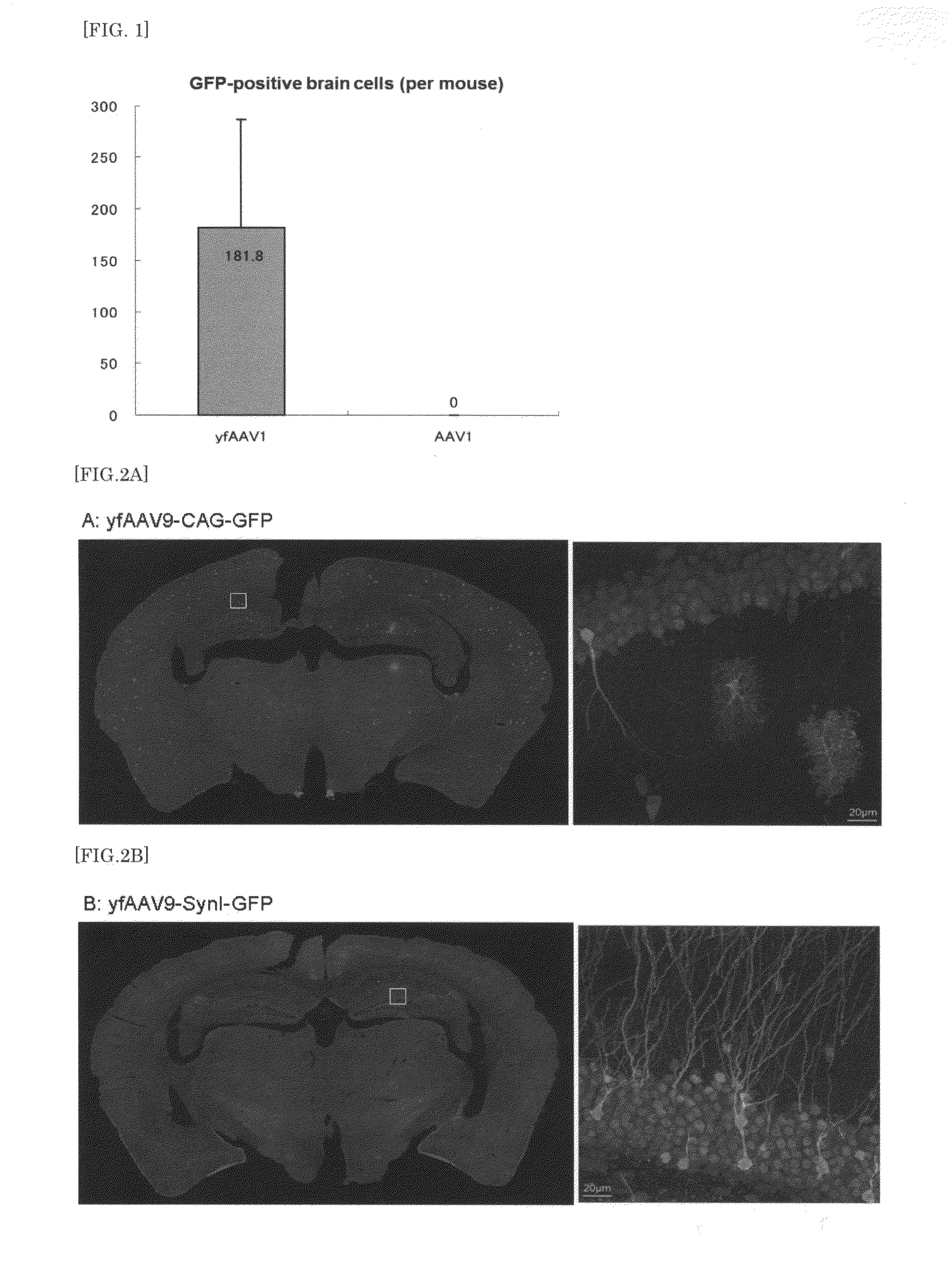
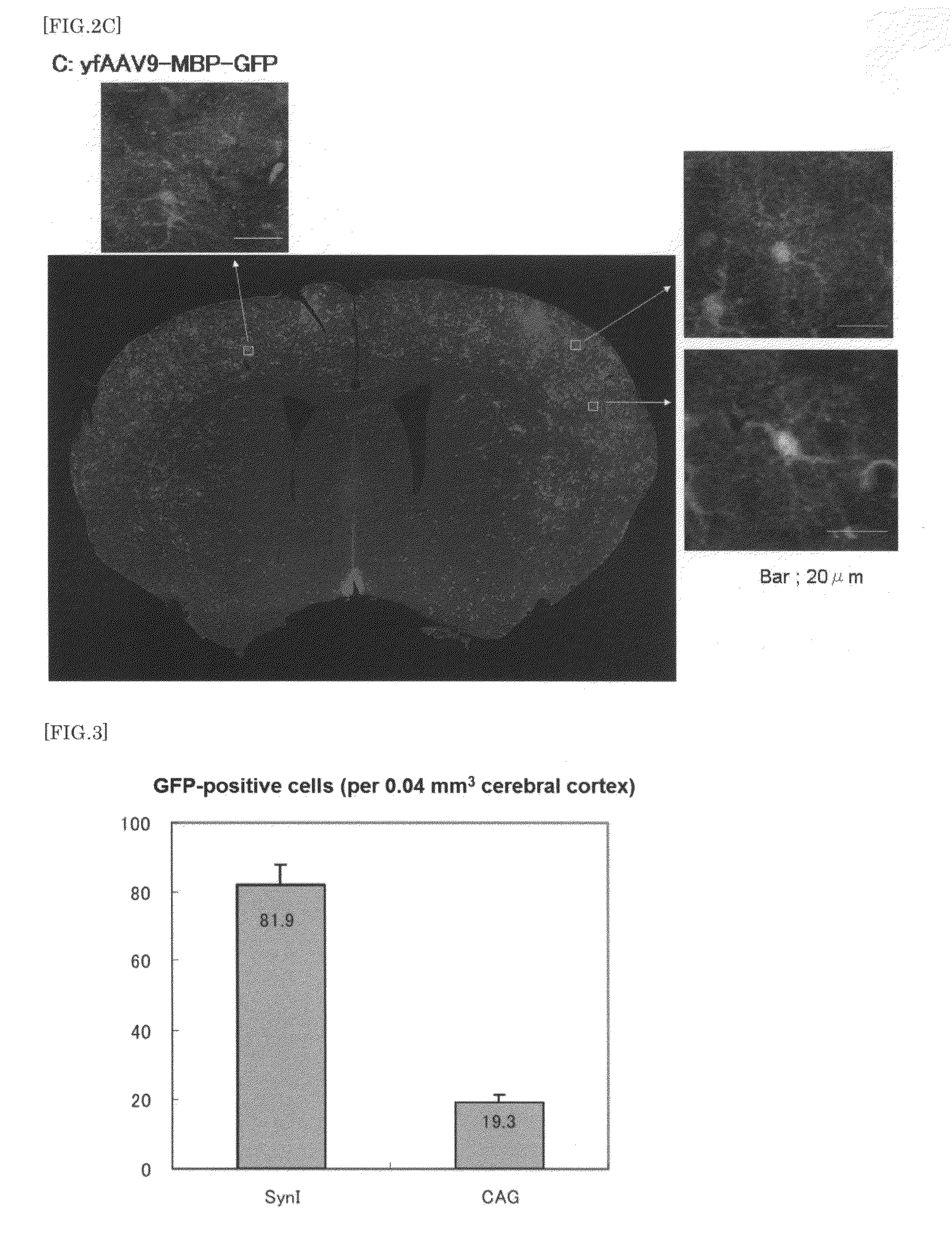
The present invention provides a means for transferring a therapeutic gene of interest into a nervous system cell by a highly-efficient and simpler means. More specifically, the present invention provides a recombinant vector that uses an adeno-associated virus (AAV), a method for manufacturing the recombinant vector, and a method for using the recombinant vector. More specifically, recombinant adeno-associated virus virions, which are capable of passing through the brain-brain barrier, for transferring a therapeutic genes of interest into a nervous system cell in a highly-efficient manner, a drug composition containing the recombinant adeno-associated virus virions, a method for manufacturing the recombinant adeno-associated virus virions, and a kit or the like are provided.
Owner:JICHI MEDICAL UNIVERSITY
Multifunctional molecular complexes for gene transfer to cells
A multifunctional molecular complex for the transfer of a nucleic acid composition to a target cell is provided The complex is comprised of A) said nucleic acid composition and B) a transfer moiety comprising 1) one or more cationic polyamines bound to said nucleic acid composition, 2) one or more endosome membrane disrupting components attached to at least one nitrogen of the polyamine and 3) one or more receptor specific binding components.
Owner:WYETH LLC
Mannose-6-phosphate receptor mediated gene transfer into muscle cells
InactiveUS20120122801A1Organic active ingredientsSpecial deliveryGlycoside formationMannose 6-phosphate receptor
The invention relates to glycoside-compound conjugates for use in antisense strategies and / or gene therapy. The conjugates comprise a glycoside linked to a compound, in which the glycoside is a ligand capable of binding to a mannose-6-phosphate receptor of a muscle cell. For example the cells are muscle cells of a Duchenne Muscular Dystrophy (DMD) patient and the conjugate comprises an antisense oligonucleotide which causes ex on skipping and induces or restores the synthesis of dystrophin or variants thereof.
Owner:PROSENSA THERAPEUTICS
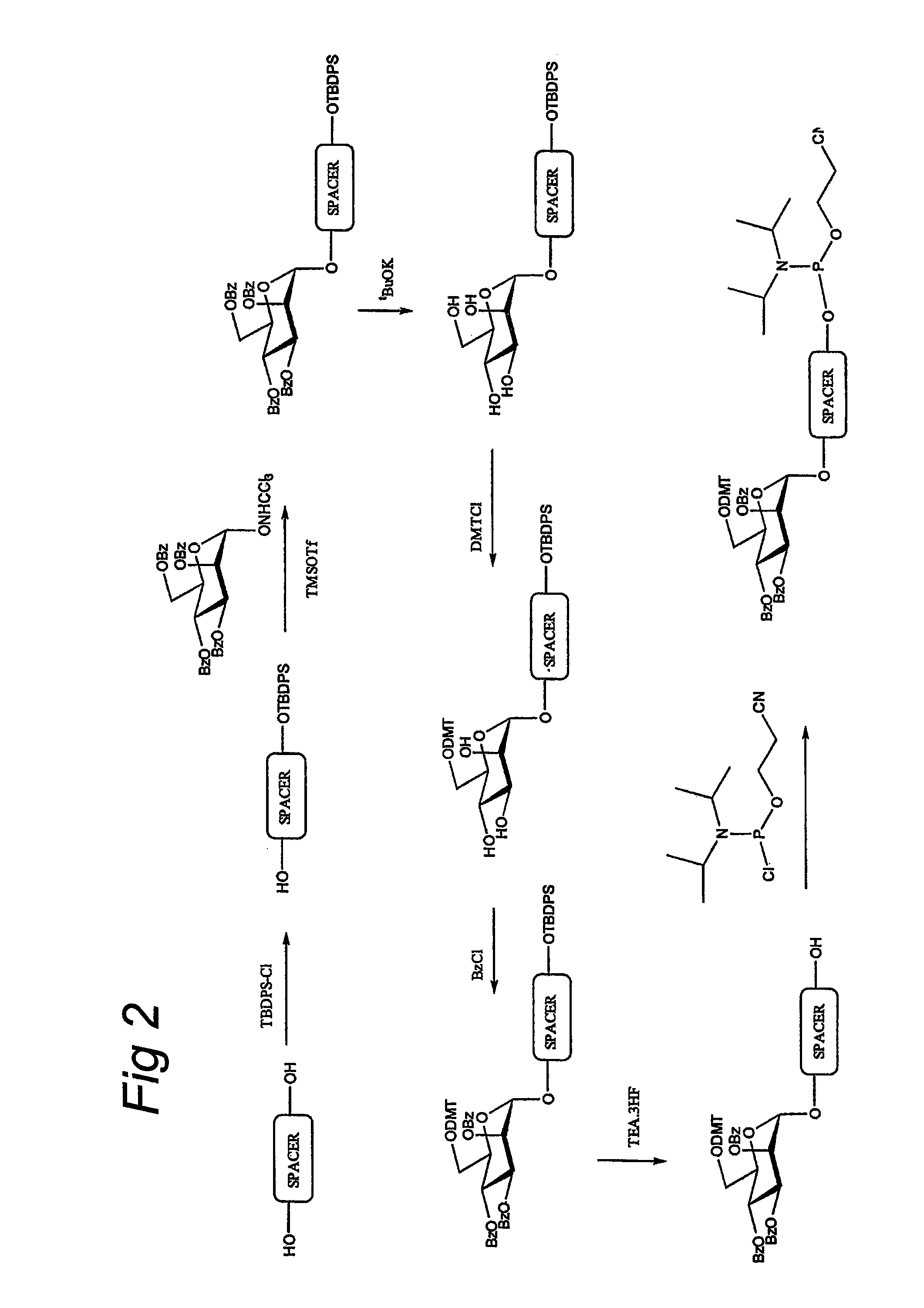
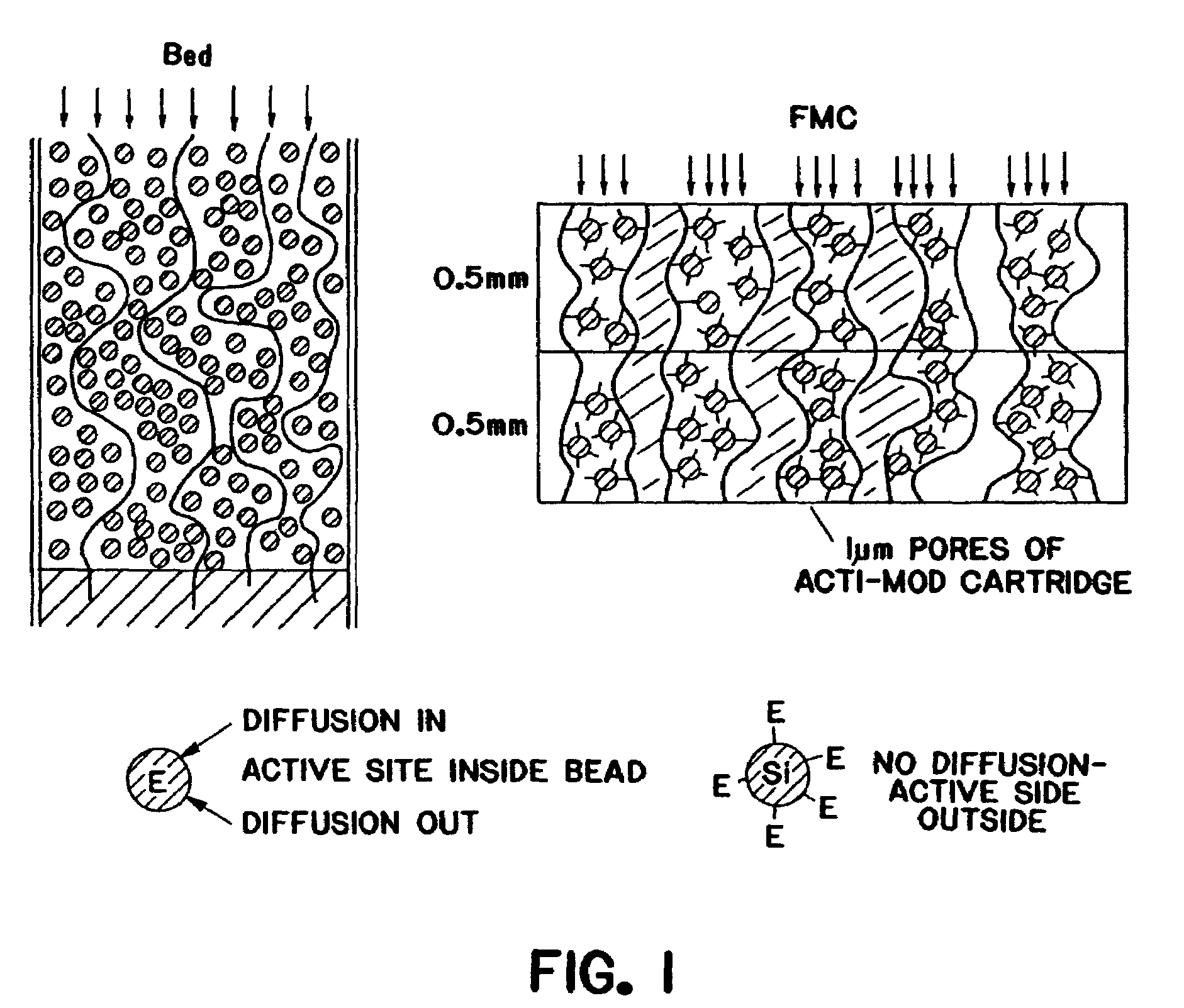
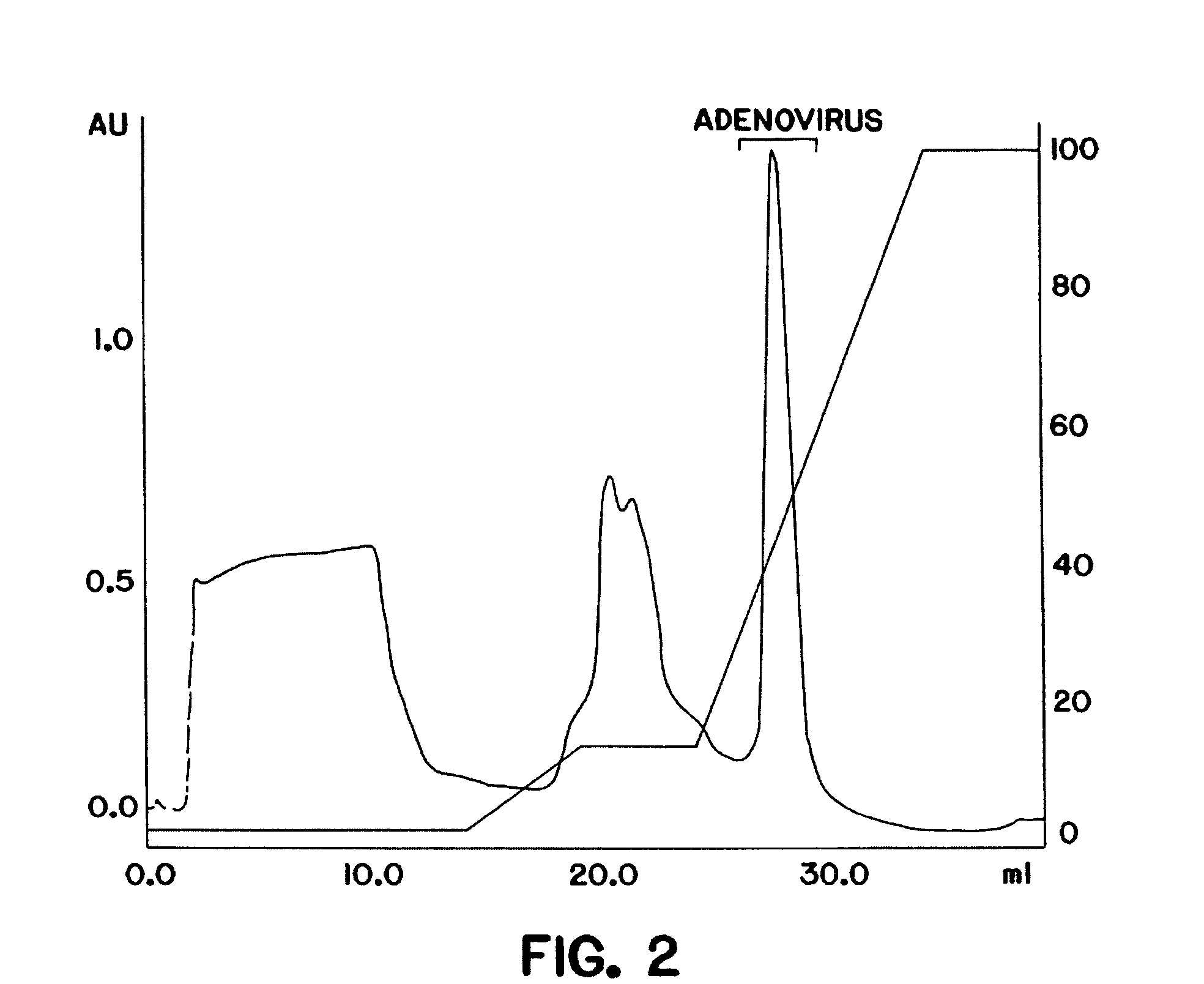
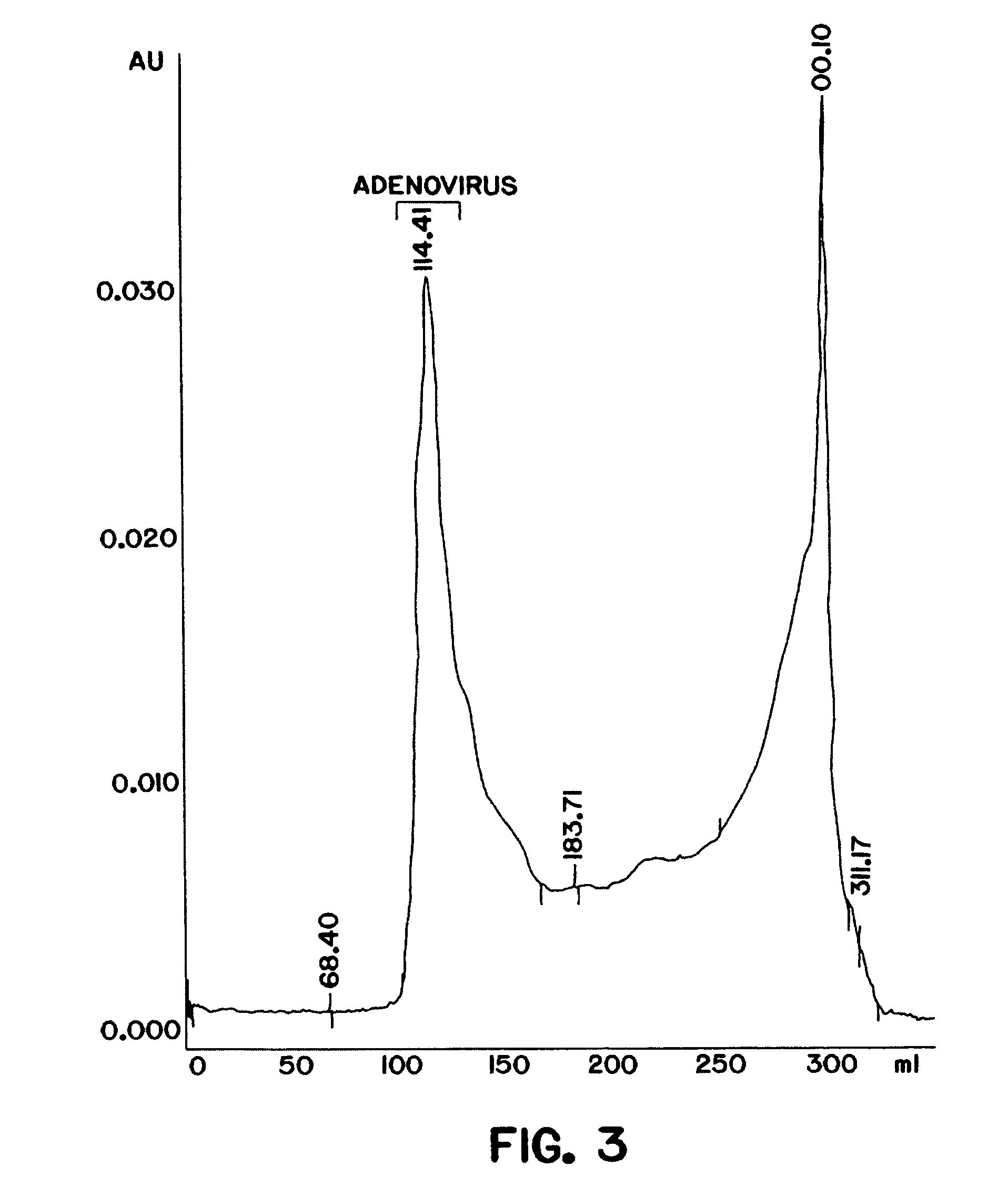
Purification of adenovirus and AAV
InactiveUS7015026B2Rapidly and efficiently purifyHigh molecular weightEnzymologyRecovery/purificationBiomedical engineeringGenetic transfer
The present invention relates to the purification of large scale quantities of active (infectious) adenovirus and AAV, especially for use in therapeutic applications. In particular, the invention provides improved methods for contacting such viruses with suitable chromatographic materials in a fashion such that any damage to the virus, particularly to surface components thereof, resulting from contact with such chromatographic materials is minimized or eliminated. The result is the ability to rapidly and efficiently purify commercial level quantities of active (infectious) virus suitable for use in therapeutic applications, e.g. gene transfer / therapy procedures.
Owner:GENZYME CORP
Drug-releasing stent with ceramic-containing layer
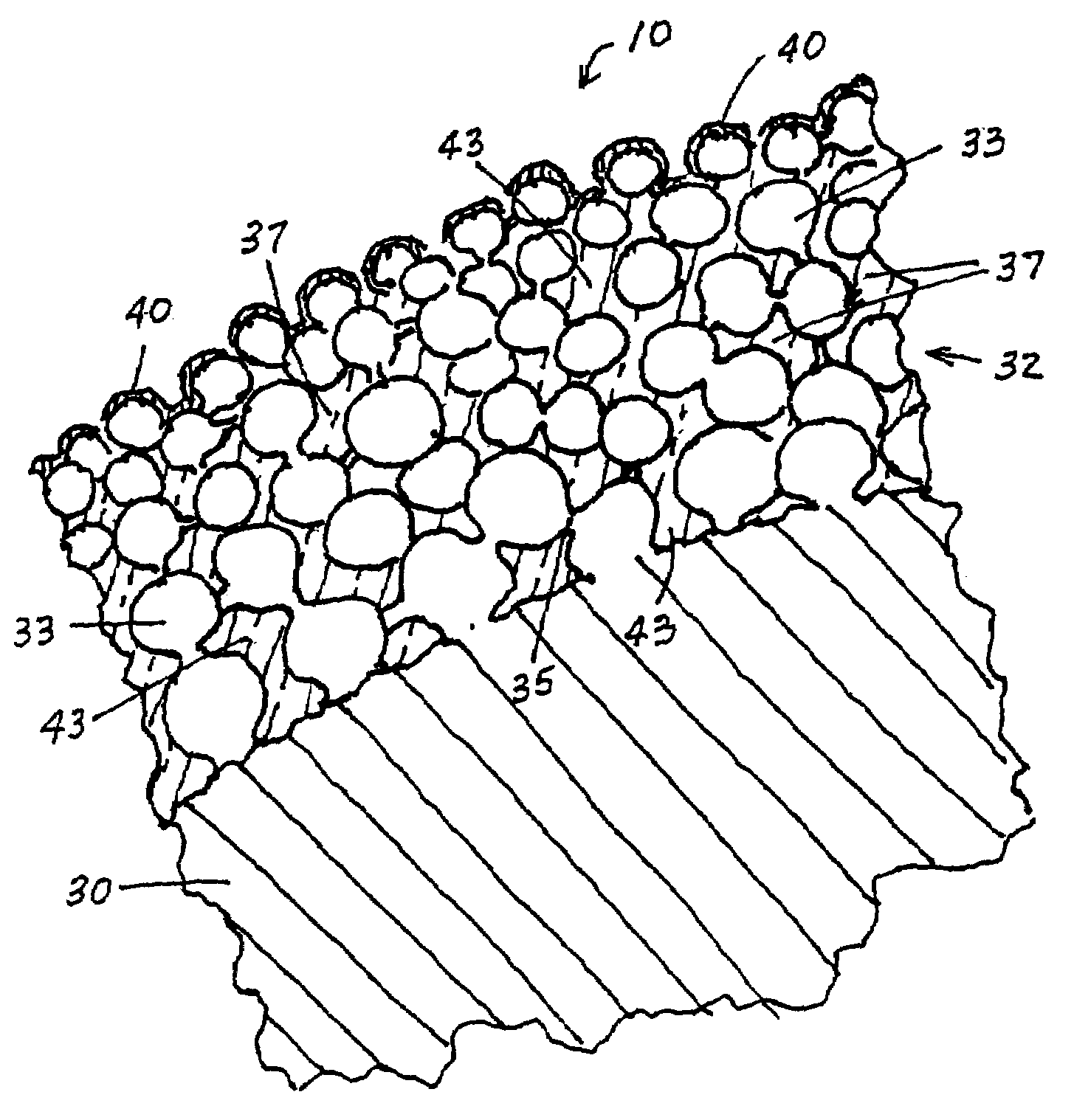
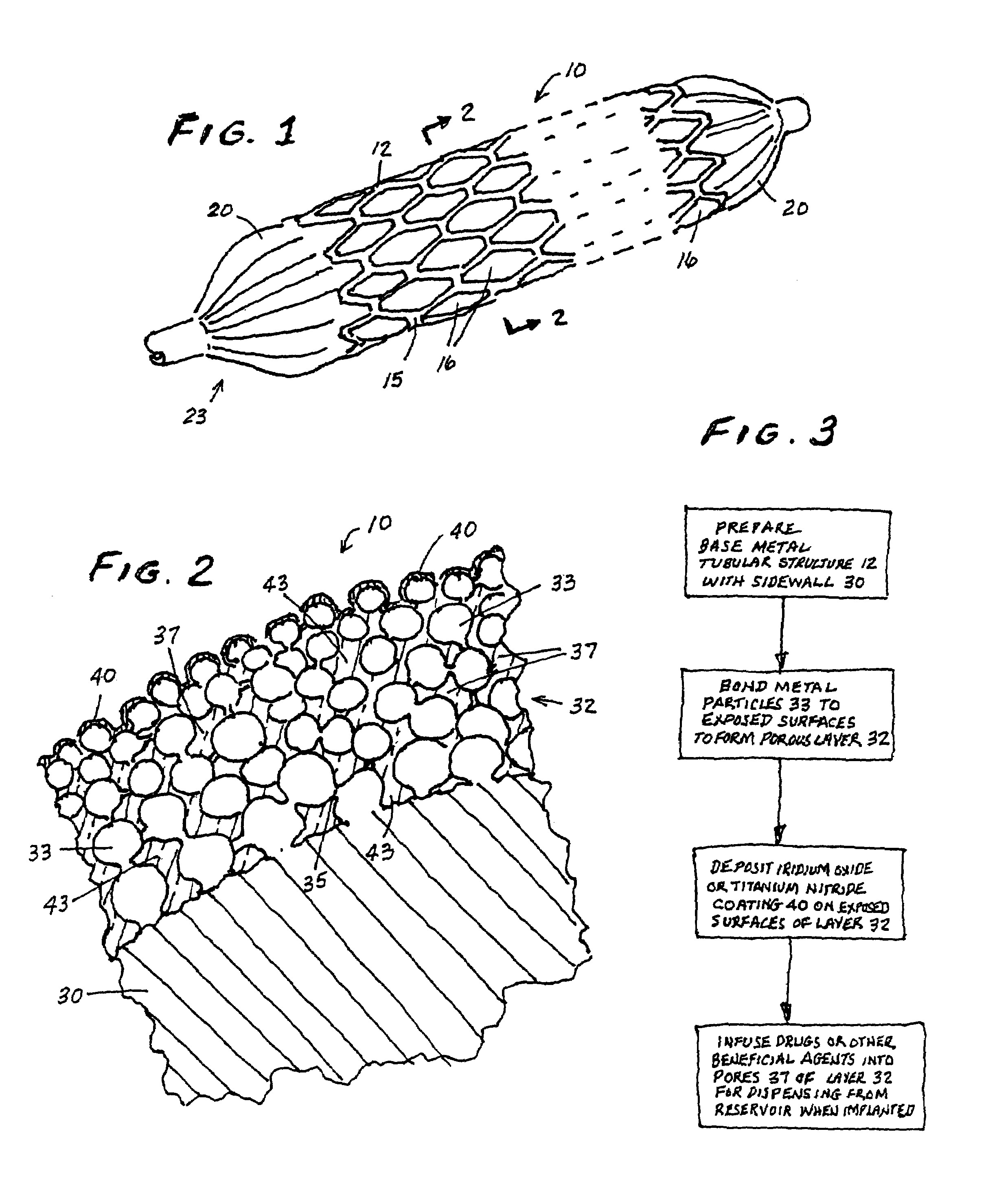
InactiveUS7713297B2Prevent proliferationPrevent restenosisStentsSurgeryEndoluminal stentAnti platelet
A vascular or endoluminal stent is adapted to be implanted in a vessel, duct or tract of a human body to maintain an open lumen at the site of the implant. The sidewall of the open-ended tubular structure of the stent is a base layer of a metal biologically compatible with blood and tissue of the human body. An intermediate metal particle layer of substantial greater radiopacity overlies the base layer, with particles bonded to the base layer and to each other to leave interstices therebetween as a repository for retaining and dispensing drugs or other agents for time release therefrom after the stent is implanted, to assist the stent in maintaining the lumen open. The particles are composed primarily of a noble metal—an alloy of platinum-iridium. The sidewall has holes extending therethrough, and the particle layer resides along the outward facing and inward facing surfaces, and the edges of the through holes and open ends of the sidewall. The larger particles are bonded to surfaces of the sidewall and progressively smaller particles are bonded to those and to each other up to the outer portion of the particle layer. Exposed surfaces of the particle layer are coated with ceramic-like iridium oxide or titanium nitrate, as a biocompatible material to inhibit irritation of tissue at the inner lining of the vessel when the stent is implanted. One or more anti-thrombotic, anti-platelet, anti-inflammatory and / or anti-proliferative drugs are retained in the interstices, together with a biodegradable carrier for time release therefrom. In an alternative embodiment, the intermediate layer is solid and the biodegradable carrier and drugs or agents therein are applied to the surface of the ceramic-like coating. Gene transfer is alternatively used to control tissue proliferation.
Owner:BOSTON SCI SCIMED INC
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
Enhanced aav-mediated gene transfer for retinal therapies
ActiveUS20150376240A1Preventing and arresting progressionPreventing and arresting of and vision lossBiocideSenses disorderAdeno associate virusRetinal treatments
Described herein are capsid proteins and adeno-associated viruses capable of targeting various types of ocular cells including bipolar and horizontal cells. Also described herein are methods of treating various ocular disorders in a subject in need thereof by administering to the subject an effective concentration of a composition comprising the recombinant adeno-associated virus (AAV) of the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Self-complementary parvoviral vectors, and methods for making and using the same
ActiveUS20070253936A1High genetic stabilityStabilized parvovirus vector is more stableBiocideAnimal repellantsGene transferTiter
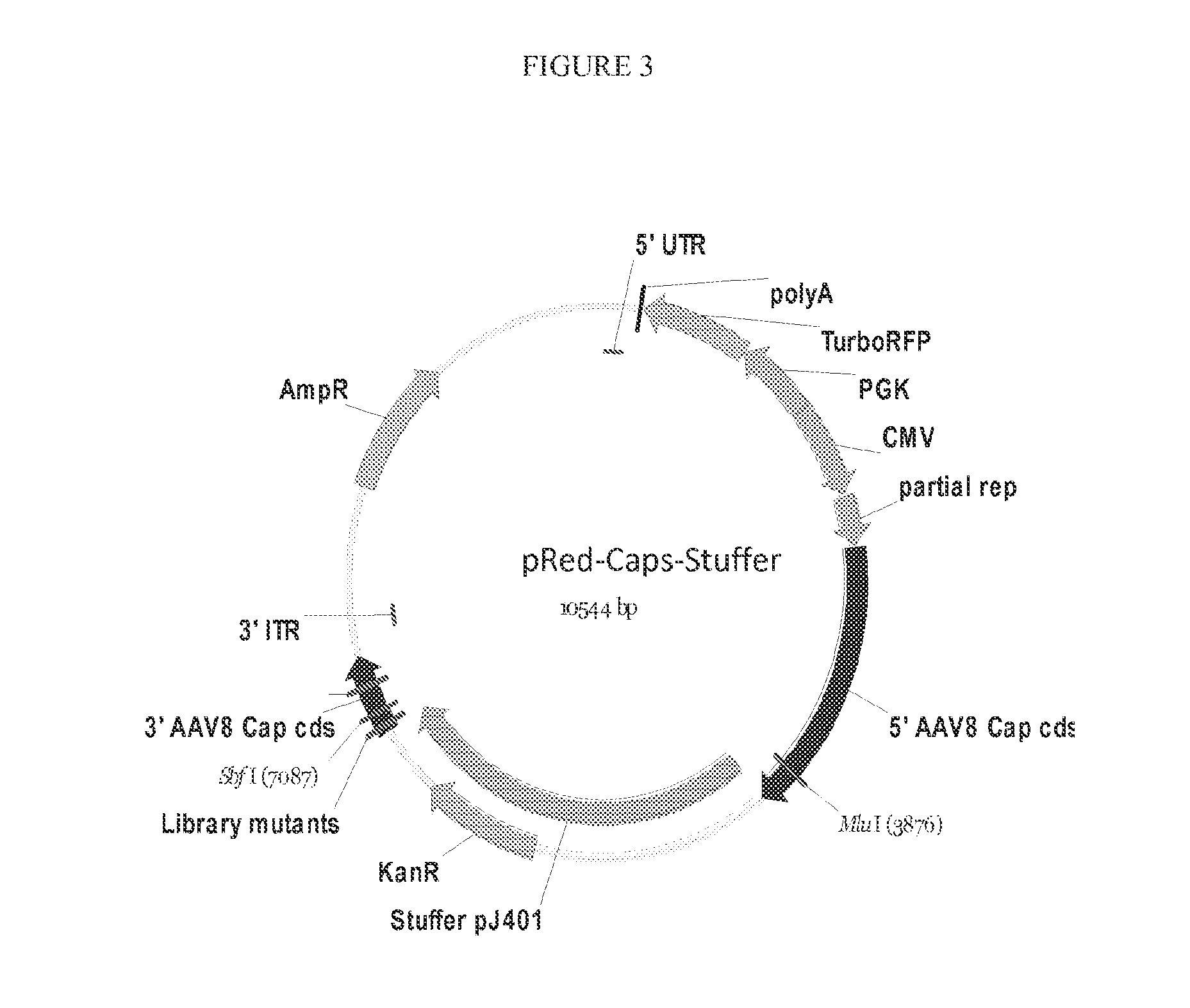
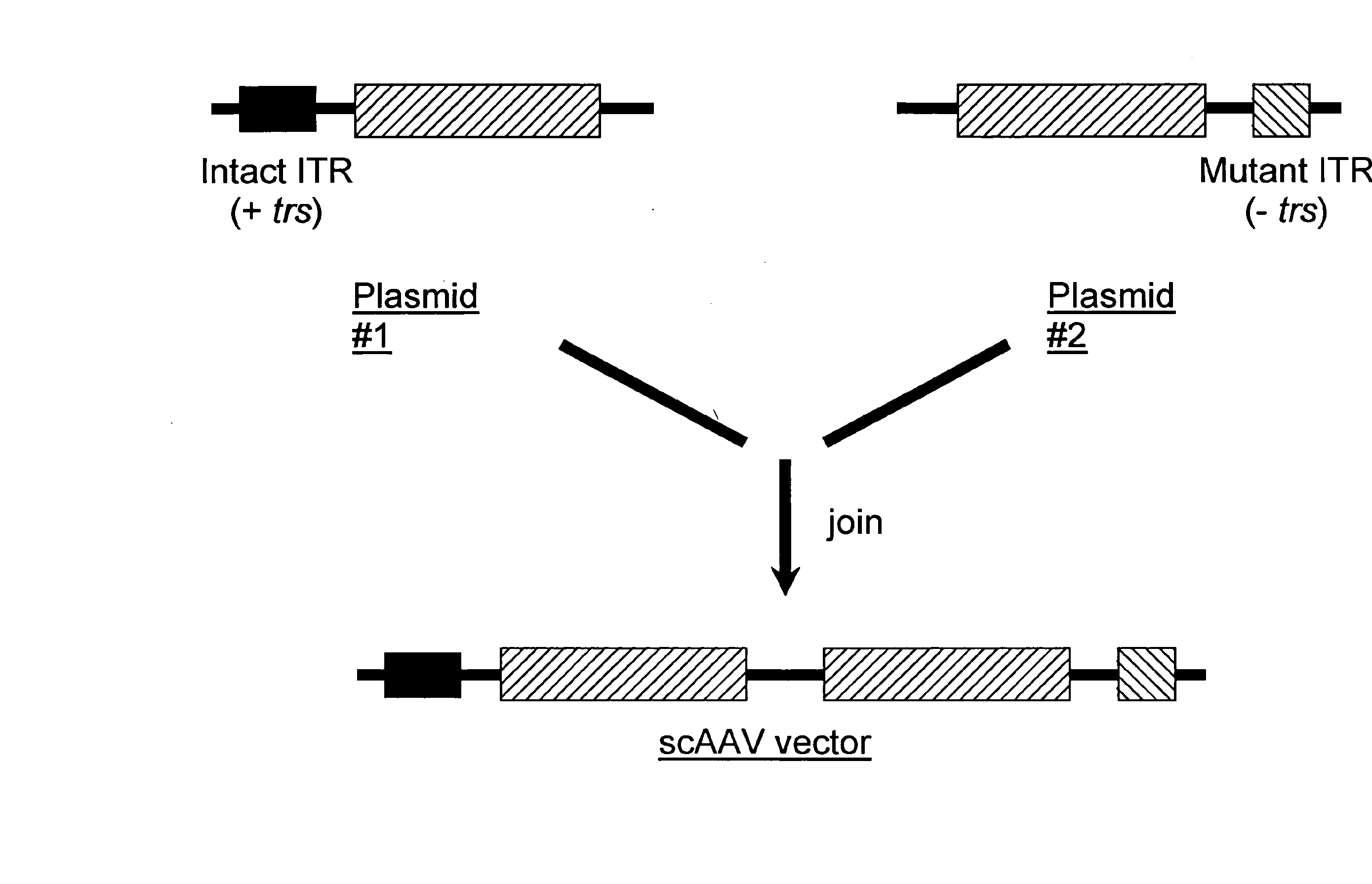
The teachings herein are generally directed to a method of enhancing the genetic stability of parvovirus vectors. The stability of conventional ss or dsAAV vector constructs can be enhanced, for example, to obtain a concurrent increase in vector titer and purity, as well as an improvement in vector safety, due at least in part to the elimination of stuffer DNA from the AAV vector. The method is broadly applicable to all gene transfer / therapy applications, such as those requiring delivery of foreign DNA containing recombinant gene expression cassettes. Such foreign DNA can range, for example, from about 0.2 up to about 5.2 kb in length. The enhanced vector constructs are highly flexible, user-friendly, and can be easily modified (via routine DNA cloning) and utilized (via standard AAV vector technology) by anyone skilled in the art.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for transposition using minimal segments of the eukaryotic transformation vector Piggybac
InactiveUS7105343B1Inserting DNA molecules into cells is enhancedEasy to insertSugar derivativesStable introduction of DNATransformation cellGene transfer
The present invention provides efficient transfer of genes into host cells or embryos to transform the cells or embryos by transposition vectors using the minimal amount of nucleotide sequences in the transposon piggyBac required for gene transfer. The transformed cells or embryos may also be developed into transgenic organisms.
Owner:UNIV OF NOTRE DAME DU LAC
Methods for modulating expression of exogenous genes in mammalian systems, and products related thereto
InactiveUS20060014711A1Precise and potent inductionImprove pharmacokineticsVectorsGenetic material ingredientsResponse elementGene transfer
In accordance with the present invention, there are provided various methods for modulating the expression of an exogenous gene in a mammalian subject employing modified ecdysone receptors. Also provided are modified ecdysone receptors, as well as homomeric and heterodimeric receptors containing sane, nucleic acids encoding invention modified ecdysone receptors, modified ecdysone response elements, gene transfer vectors, recombinant cells, and transgenic animals containing nucleic acids encoding invention modified ecdysone receptor.
Owner:EVANS RONALD +2
Fixed-point gene editing method based on intratestis injection
InactiveCN105296537AVector-based foreign material introductionAnimal husbandryMatingGene Modification
The invention discloses a simple, convenient and efficient fixed-point gene editing method relating to the fields of transgenic animal preparation, gene functional study and biomedicines. A testis injection method is a sperm-mediated gene transferring method developed by utilizing the sperm capacity of actively combining, transferring and integrating an exogenous DNA. By using the method disclosed by the invention, an animal testis injection method is optimized, a carrier constructed by utilizing a clustered regularly interspaced short palindromic repeat system (CRISPR / Cas9) is integrated with a sperm chromosome through multi-point testis injection or seminiferous tubule microinjection, so that the aims of deleting, replacing and inserting genes are achieved. Then, a transgenic animal descendant is obtained through various approaches such as natural mating and artificial insemination. According to the method, the advantages of a CRISPR / Cas9 gene editing system are sufficiently utilized, and the existing transgenic animal system is combined, so that the complexity of in-vitro cell operation and requirement on expensive precise instruments / equipment are avoided while the gene modification animal obtaining efficiency is greatly increased.
Owner:SOUTHWEST UNIVERSITY
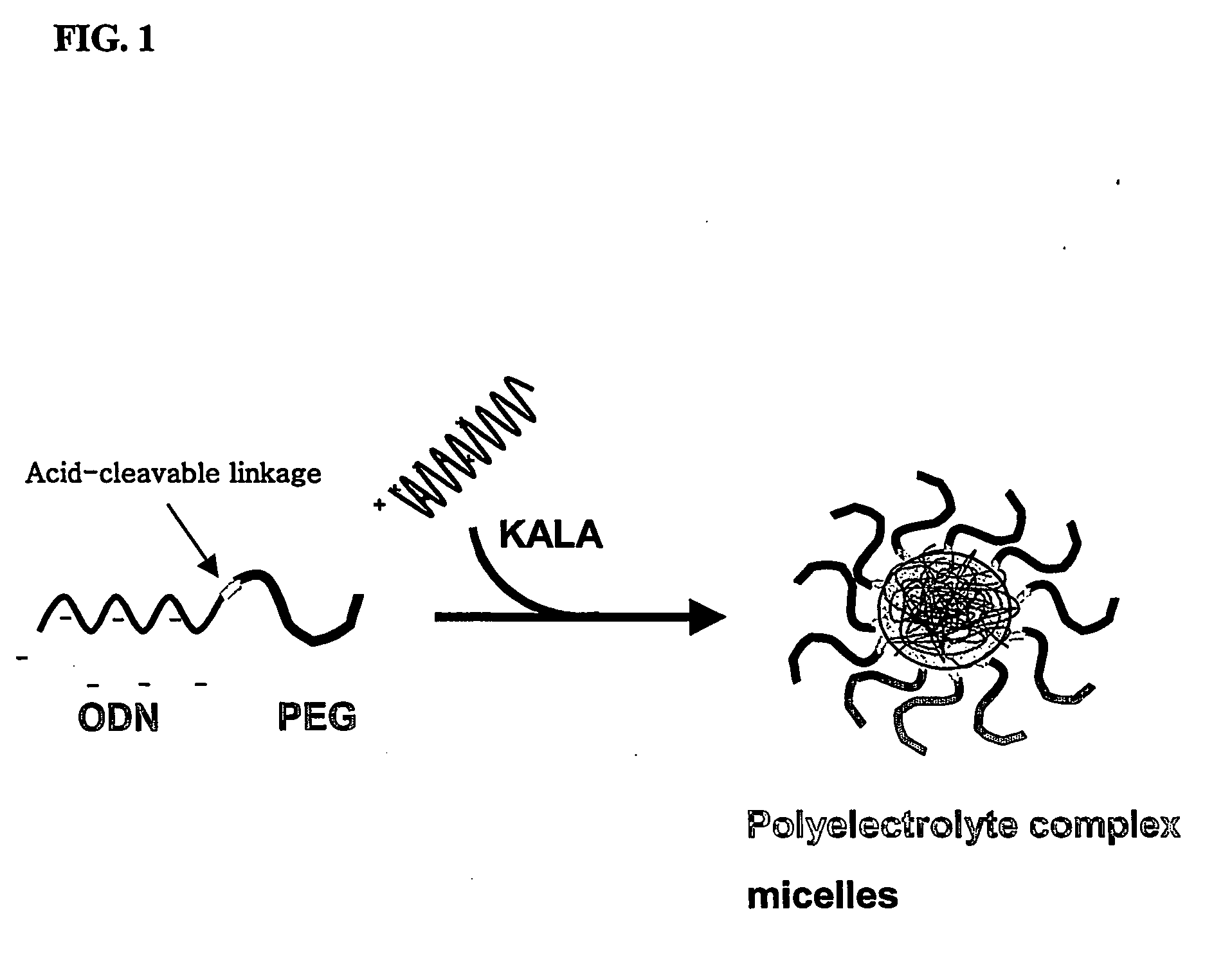
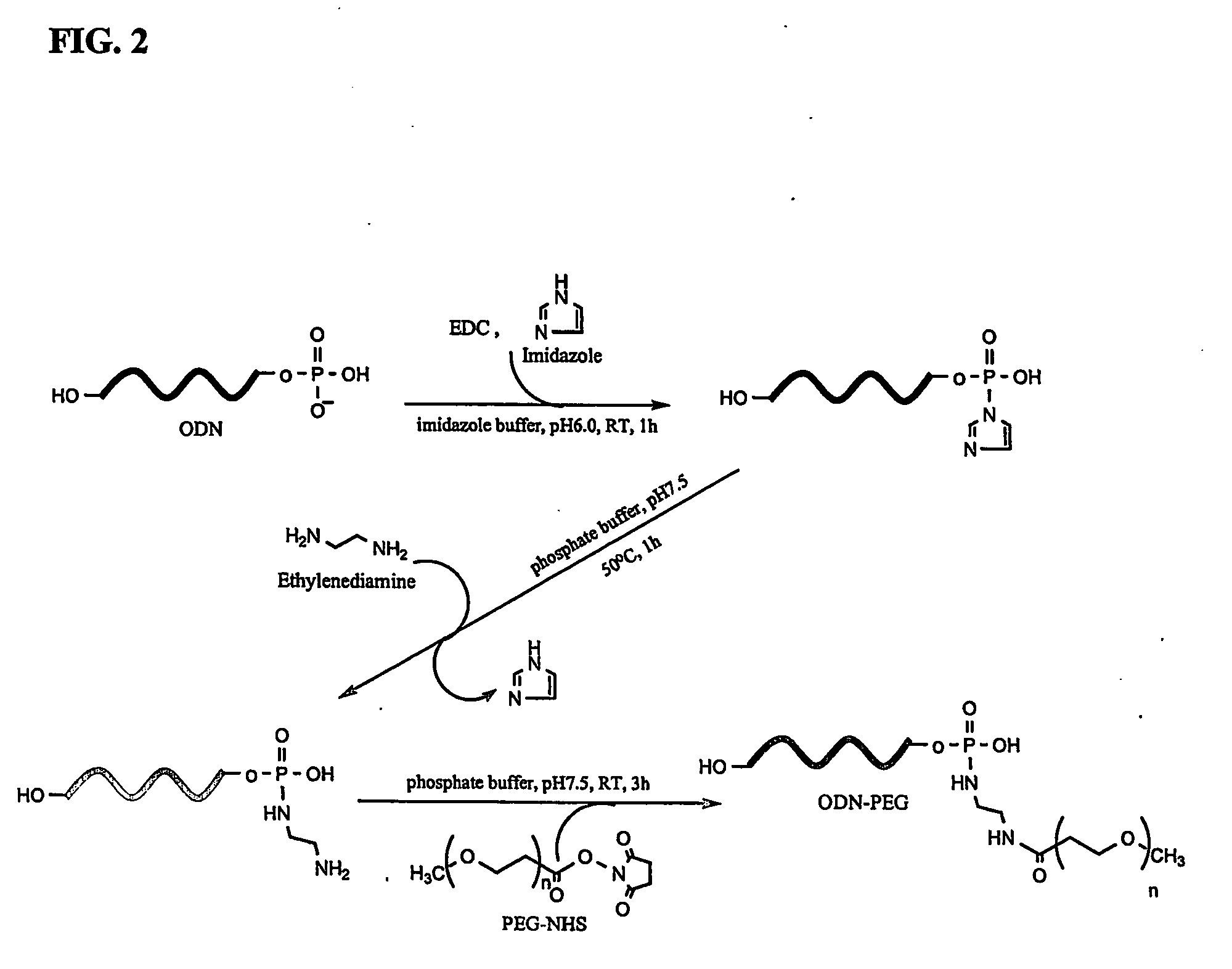
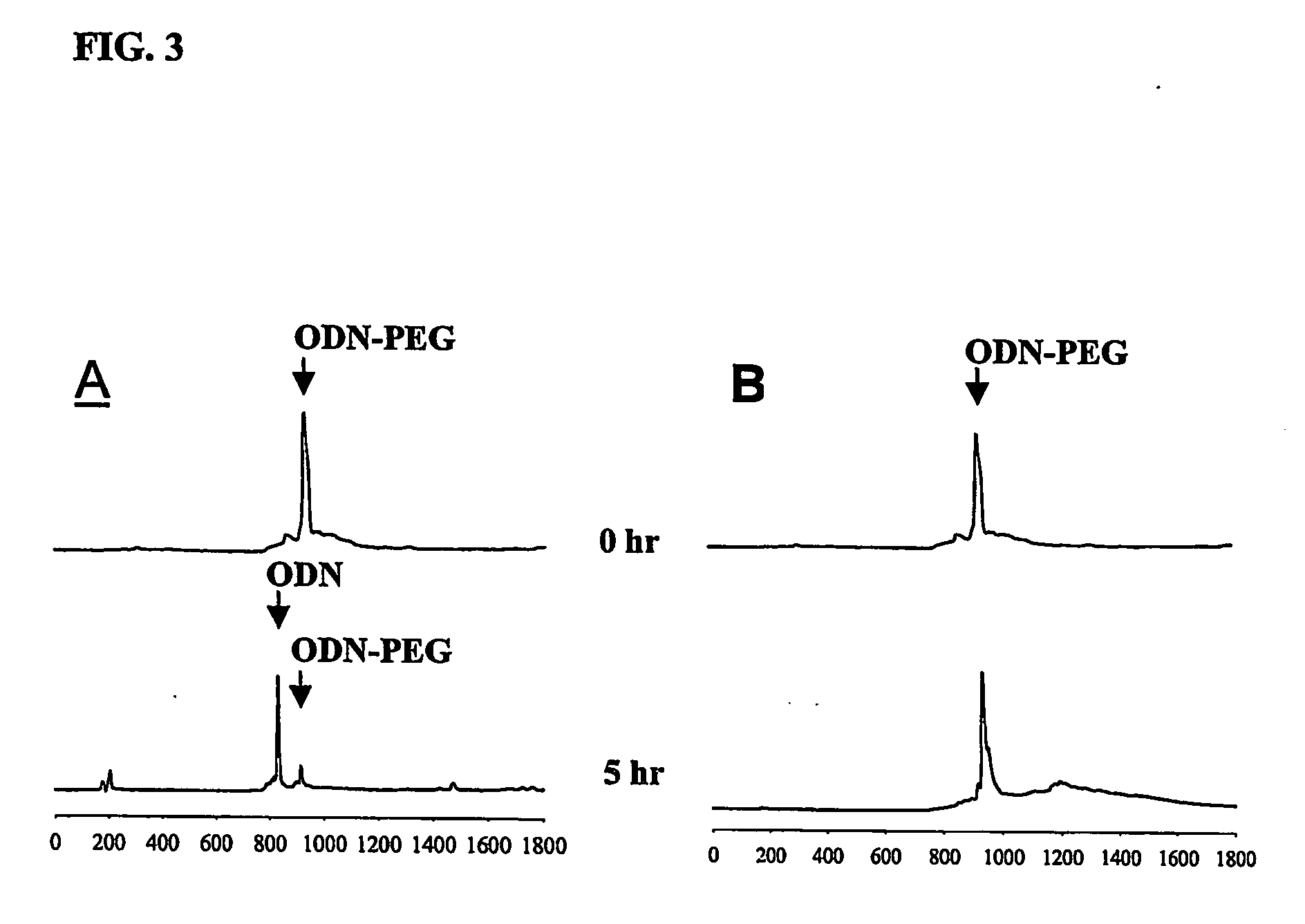
Conjugate for gene transfer comprising oligonucleotide and hydrophilic polymer, polyelectrolyte complex micelles formed from the conjugate, and methods for preparation thereof
ActiveUS20070041932A1Enhance intracellular deliveryGood biocompatibilityOrganic active ingredientsSugar derivativesIncurable diseasesPolyelectrolyte
Disclosed is a conjugate for gene transfer, which is capable of being used for treatment of incurable diseases, comprising an oligonucleotide intended to be transferred into target cells and a hydrophilic polymer, wherein an end of the oligonucleotide is covalently conjugated to the hydrophilic polymer. Also, the present invention discloses polyelectrolyte complex micelles formed from such a conjugate and a cationic polymer or cationic peptide. Such polyelectrolyte complex micelles can effectively transfer oligonucleotides as therapeutic agents into target cells, making it possible to obtain desired activities of the delivered oligonucleotides in target cells even when the micelles are clinically applied at a relatively low concentration. Therefore, the conjugate and the polyelectrolyte complex micelle are very useful in basic life science research and the medical field.
Owner:BIONEER CORP 33 3 +3
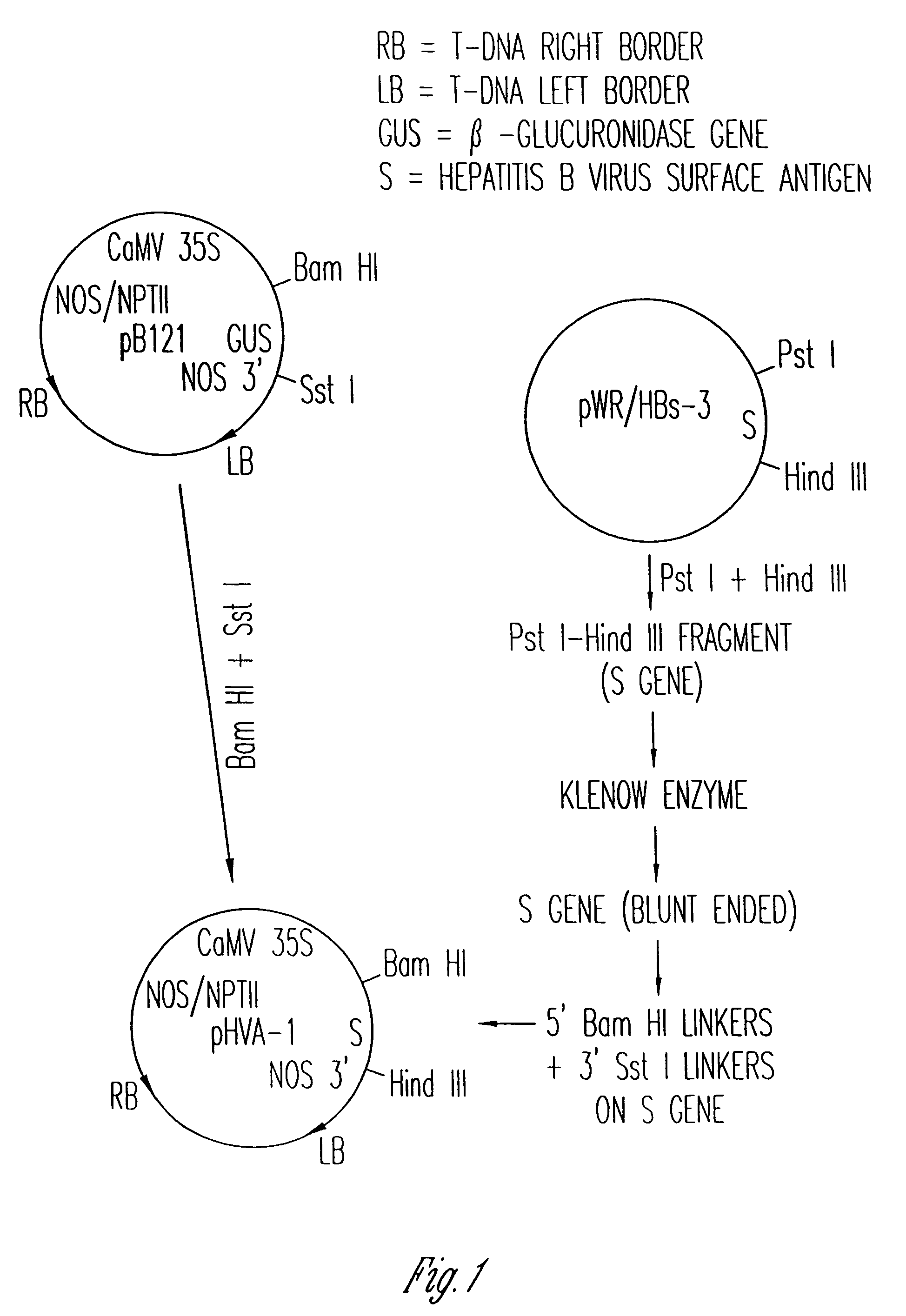
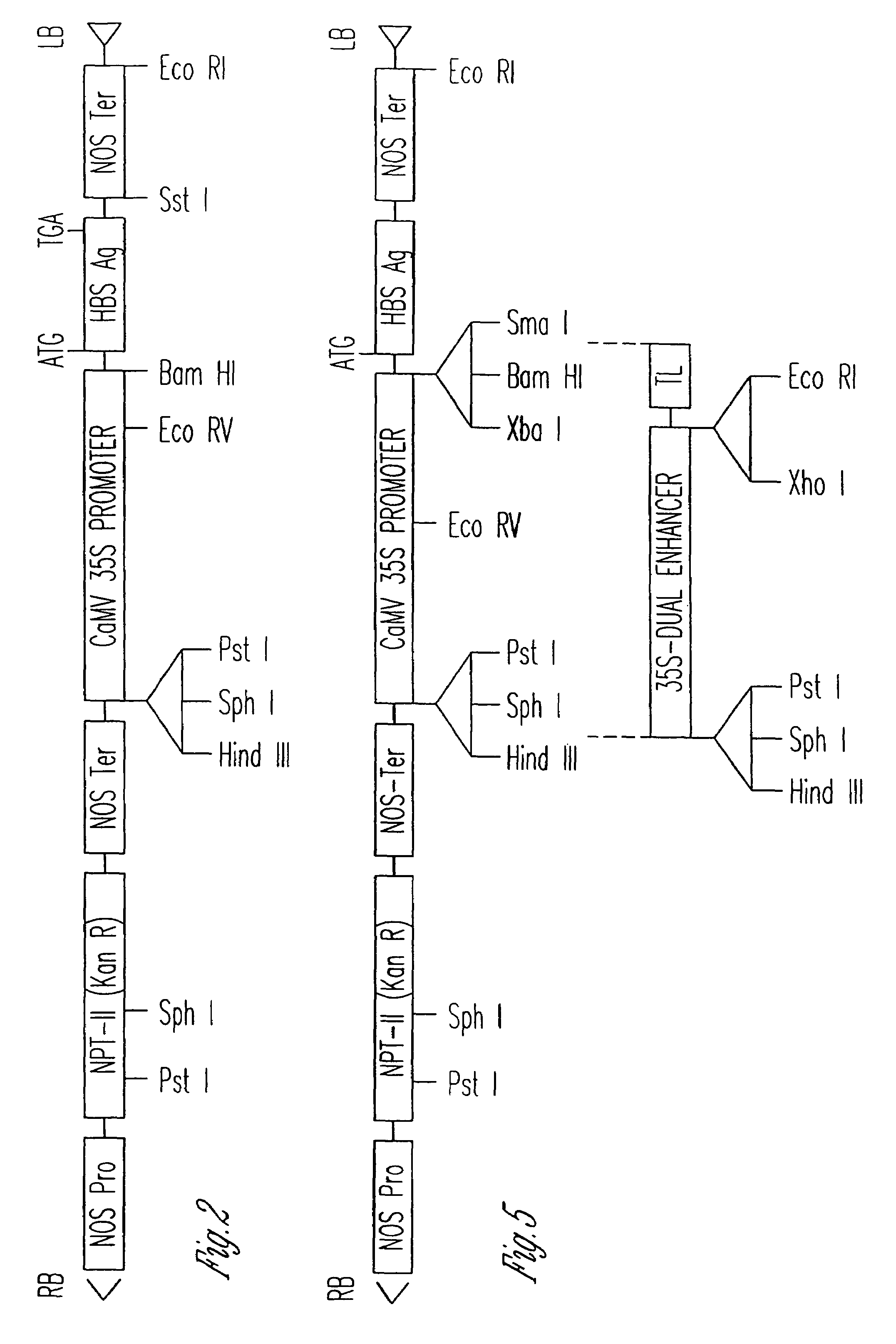
Vaccines expressed in plants
The anti-viral vaccine of the present invention is produced in transgenic plants and then administered through standard vaccine introduction method or through the consumption of the edible portion of those plants. A DNA sequence encoding for the expression of a surface antigen of a viral pathogen is isolated and ligated to a promoter which can regulate the production of the surface antigen in a transgenic plant. This gene is then transferred to plant cells using a procedure that results in its integration into the plant genome, such as through the use of an Agrobacterium tumenfaciens plasmid vector system. Preferably, the foreign gene is expressed in an portion of the plant that is edible by humans or animals. In a preferred procedure, the vaccine is administered through the consumption of the edible plant as food, preferably in the form of a fruit or vegetable juice which can be taken orally.
Owner:PRODI GENE INC
Expression cloning processes for the discovery characterization, and isolation of genes encoding polypeptides with a predetermined property
InactiveUS6197502B1Protein production is suppressedSugar derivativesMicrobiological testing/measurementBinding siteRapid identification
The present invention is directed to a method for detection, characterization and isolation of nucleic acids encoding proteins of a desired property, such as a particular cellular localization. The invention further provides for rapid expression of such proteins or glycoproteins in mammalian cells and for facilitated purification of the novel secreted proteins or glycoproteins. Further, the invention provides for radioactive labelling of the novel proteins or glycoproteins, for rapid identification of sites of binding including animals and for rapid production of infective viral vectors for use in gene transfer.
Owner:CYTOS BIOTECHNOLOGY AG
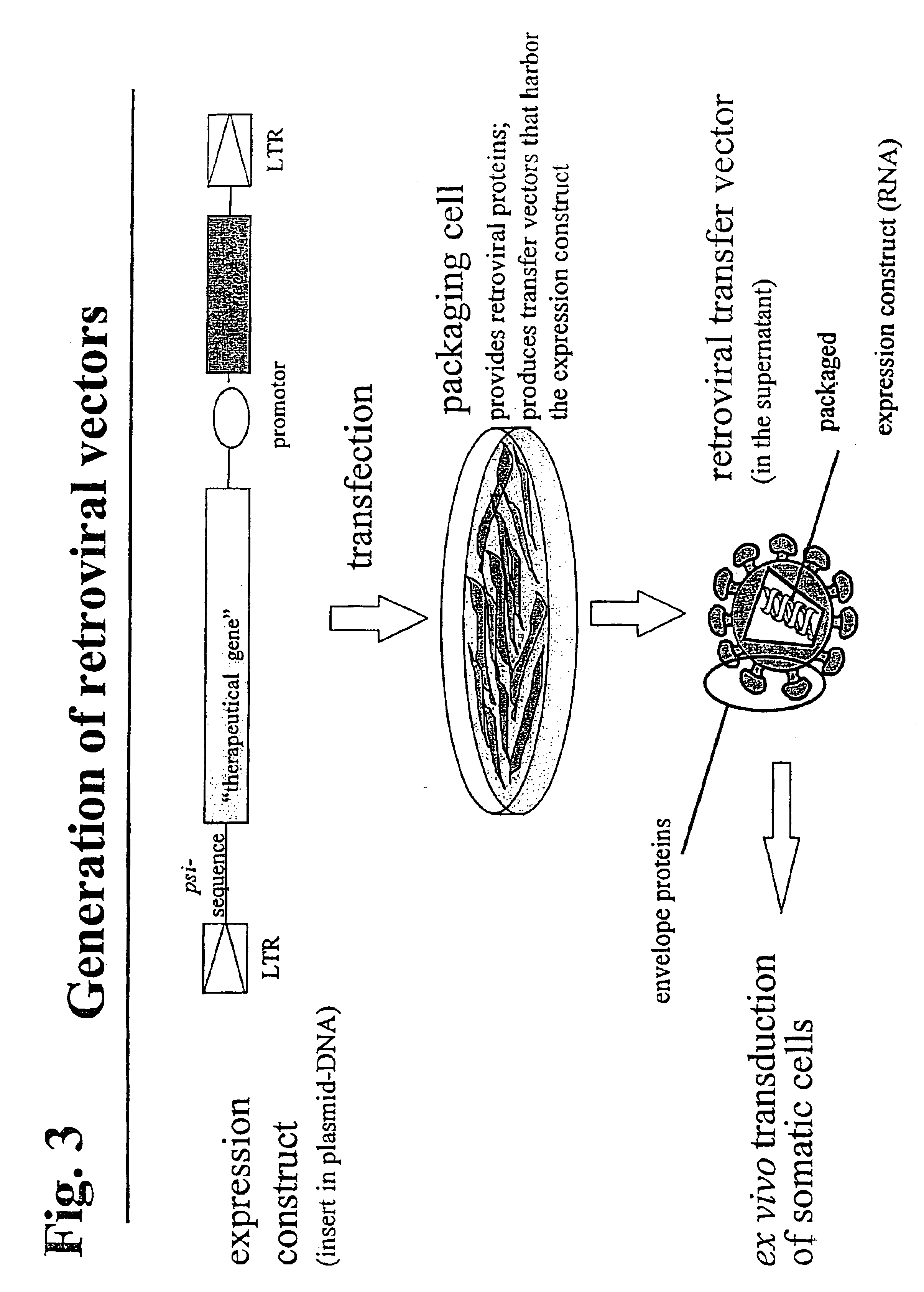
Enhanced nucleic acid constructs for eukaryotic gene expression
ActiveUS9428767B2High expressionReduce interferencePolypeptide with localisation/targeting motifOrganic active ingredientsHeterologousEukaryotic gene
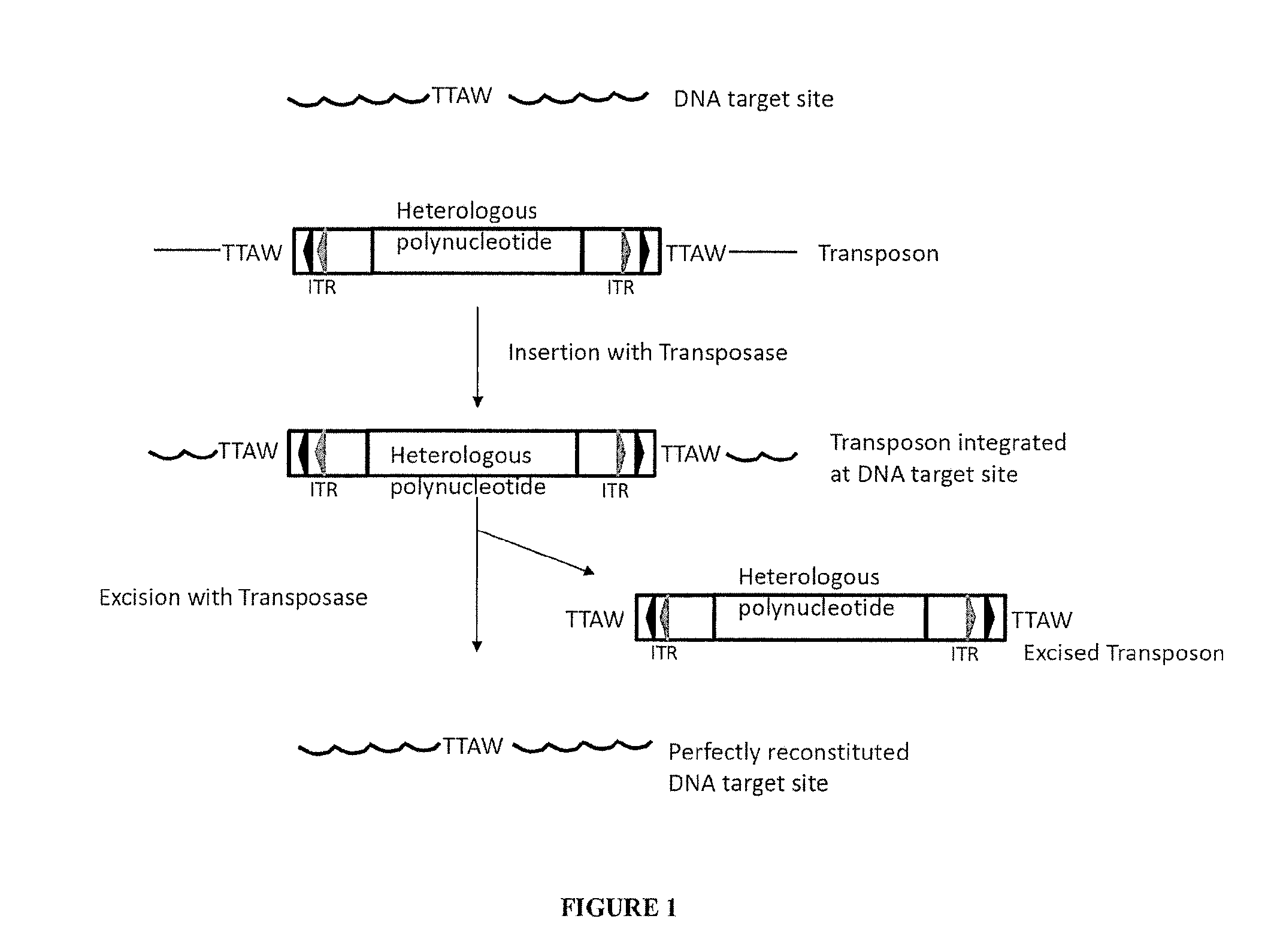
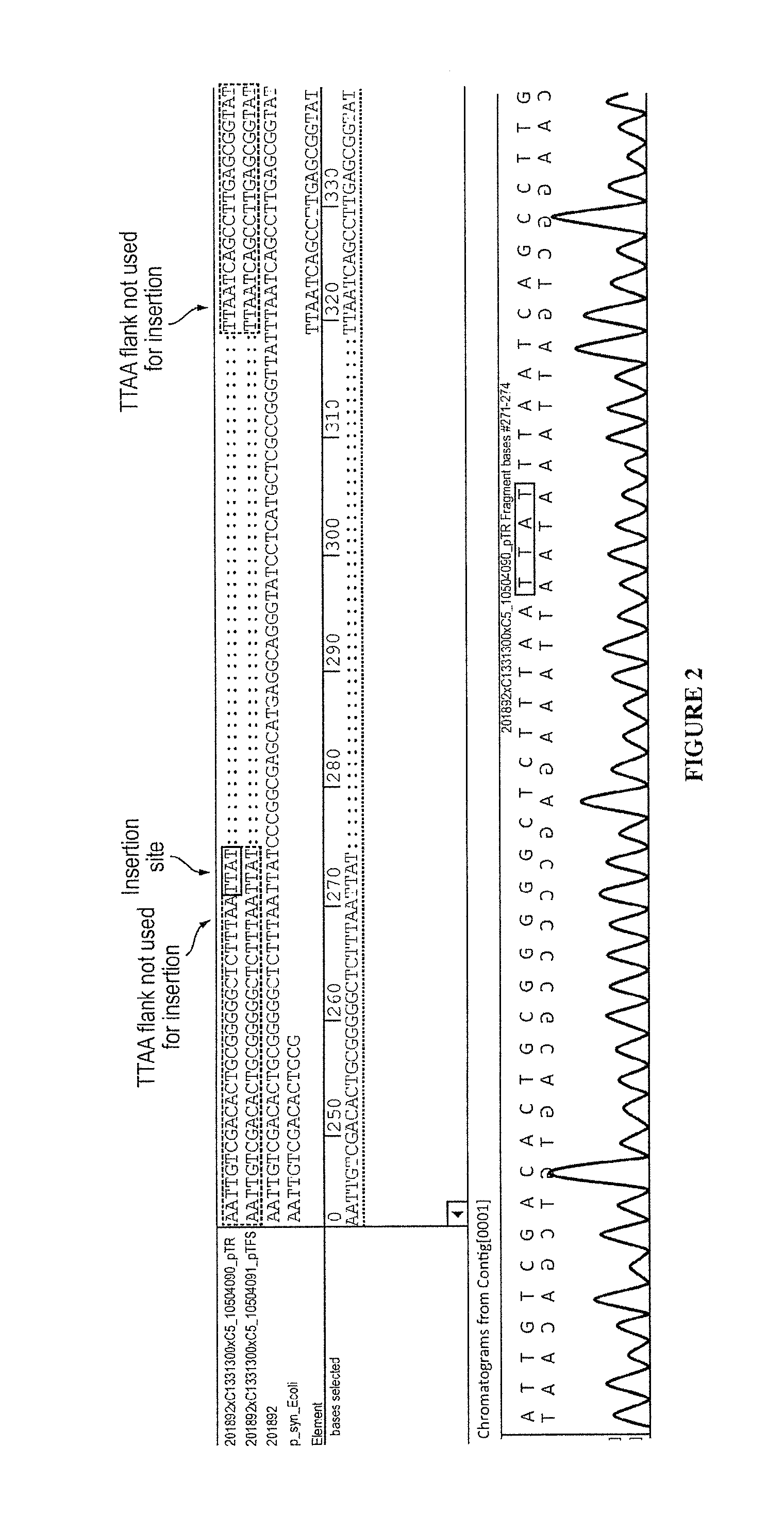
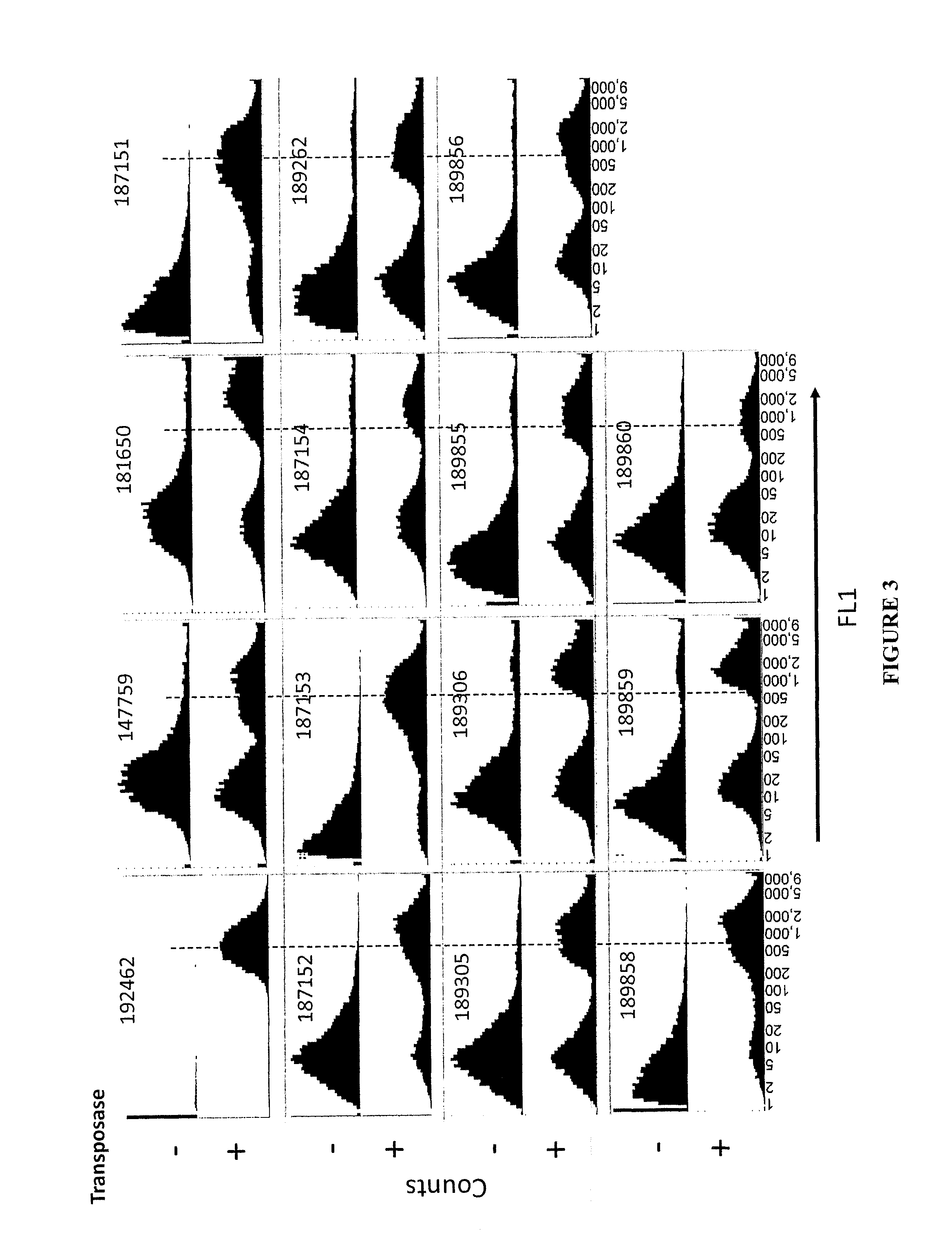
The present invention provides polynucleotide vectors for high expression of heterologous genes, and methods for constructing such vectors. Some vectors further comprise novel transposons and transposases that further improve expression. Further disclosed are vectors that can be used in a gene transfer system for stably introducing nucleic acids into the DNA of a cell. The gene transfer systems can be used in methods, for example, but not limited to, gene expression, gene therapy, insertional mutagenesis, or gene discovery.
Owner:DNA2 0
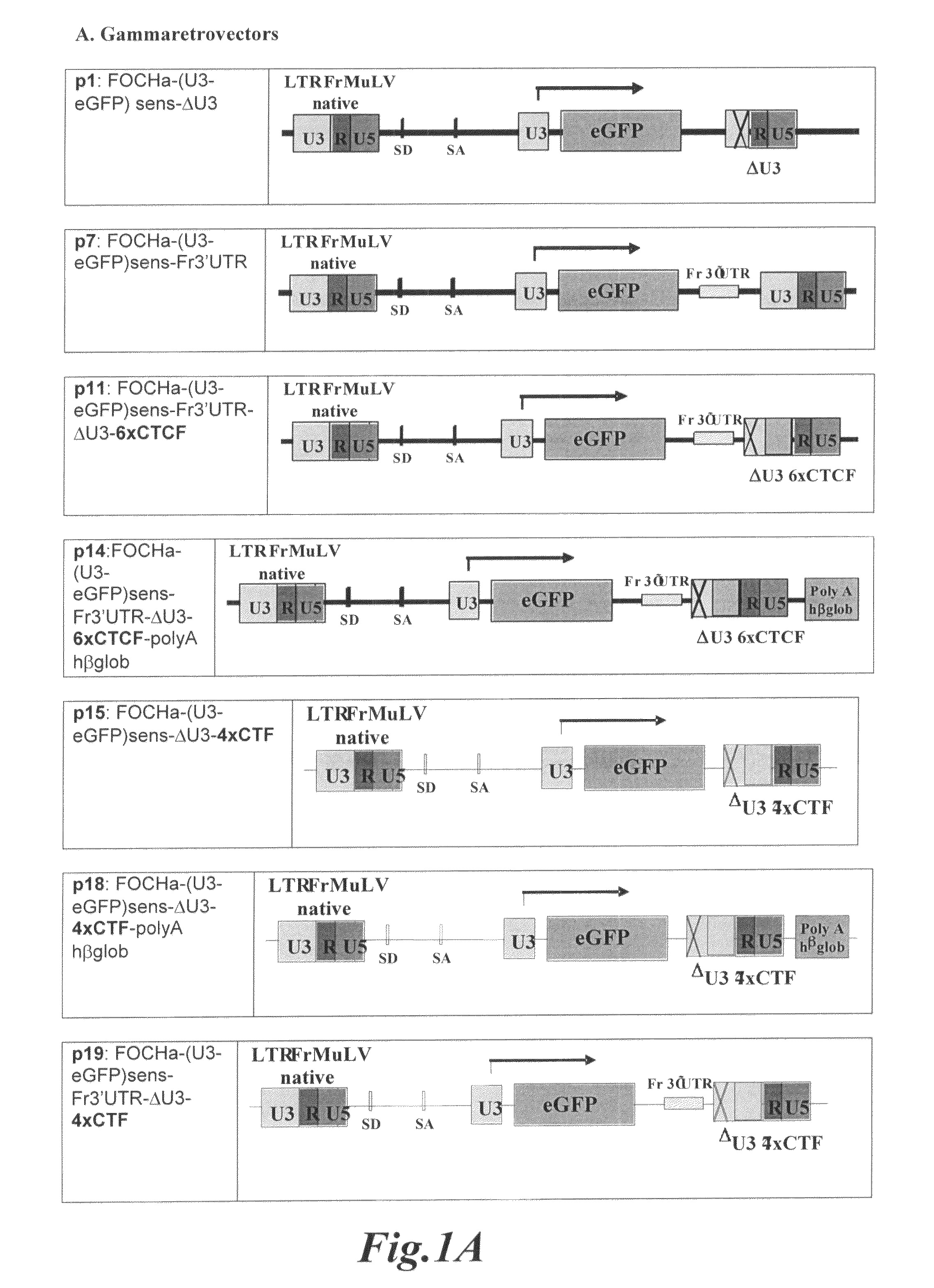
Gene transfer vectors comprising genetic insulator elements and methods to identify genetic insulator elements
ActiveUS8828718B2Reduced expression levelVectorsGenetic material ingredientsBiotechnologyBinding site
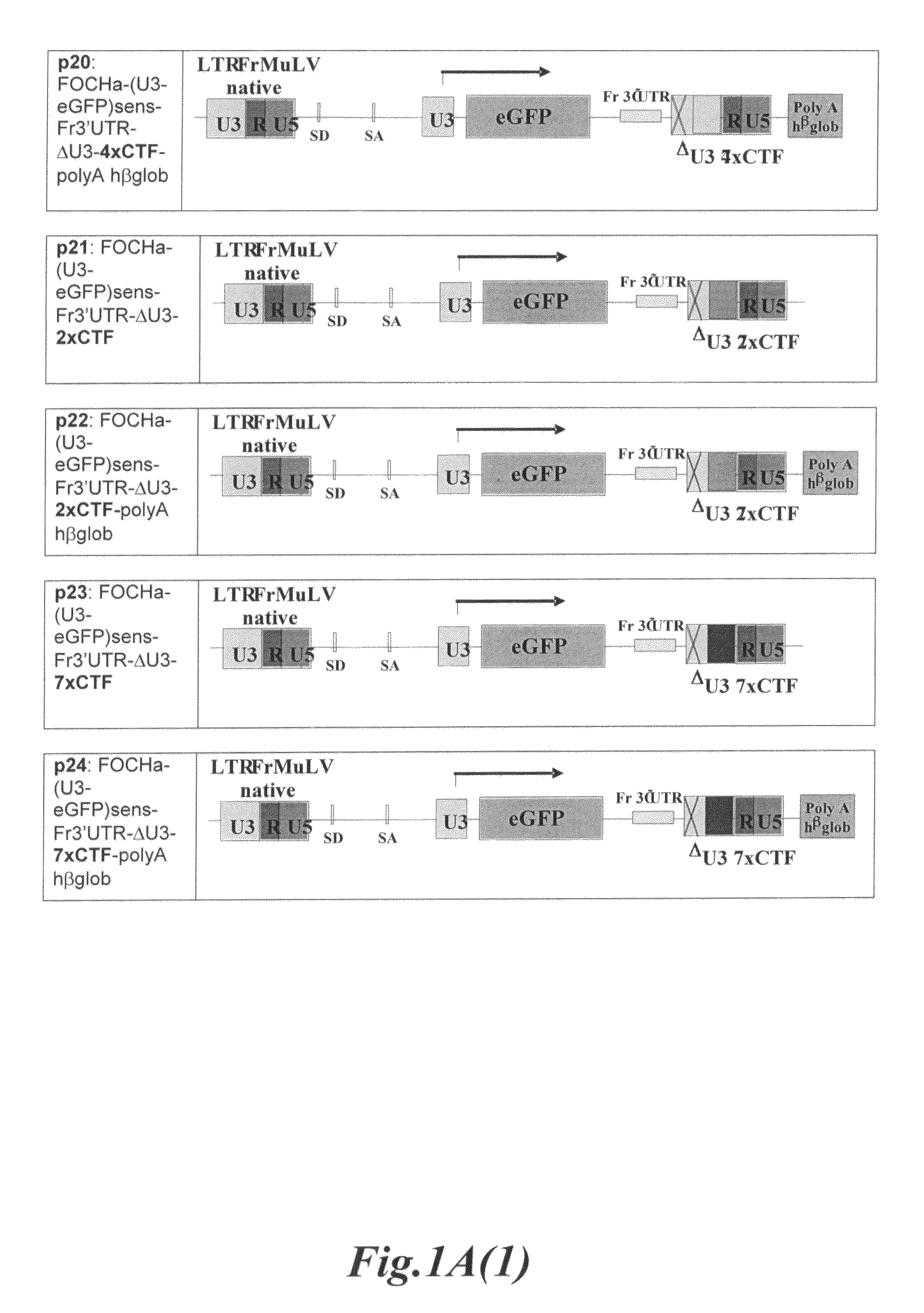
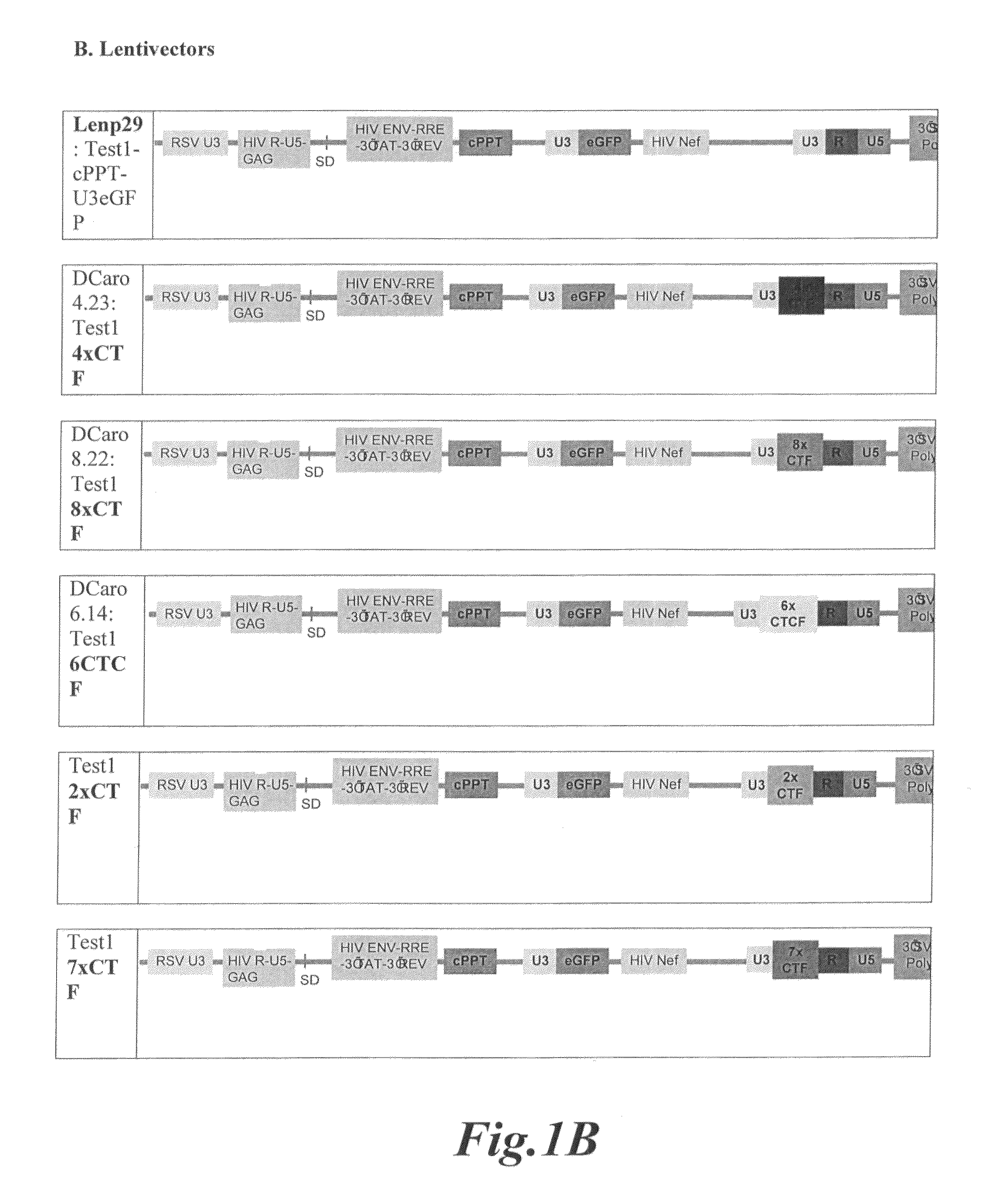
The present invention relates to a gene transfer vector (GTV) and in particular to an integrating gene transfer vector (IGTV), which comprises at least one genetic insulator element (GIE), wherein the each comprises at least two copies of an element selected from the group consisting of: a CTF binding site; a first CTCF binding site and a second CTCF binding site, wherein the first and the second CTCF binding sites are derived from the regulatory sequences of different genes.
Owner:CENT NAT DE LA RECHERCHE SCI
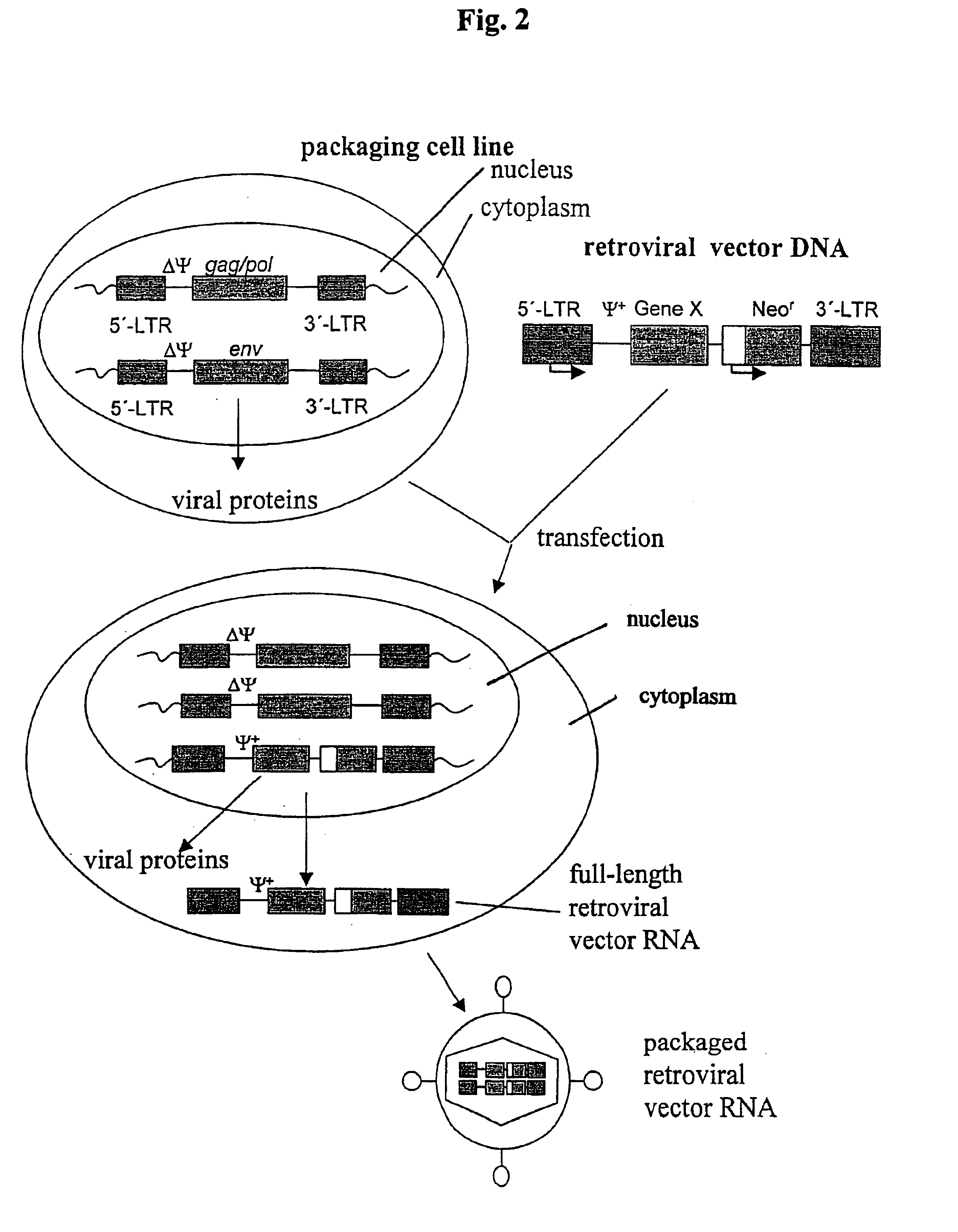
Retrovirus vectors derived from avian sarcoma leukosis viruses permitting transfer of genes into mammalian cells
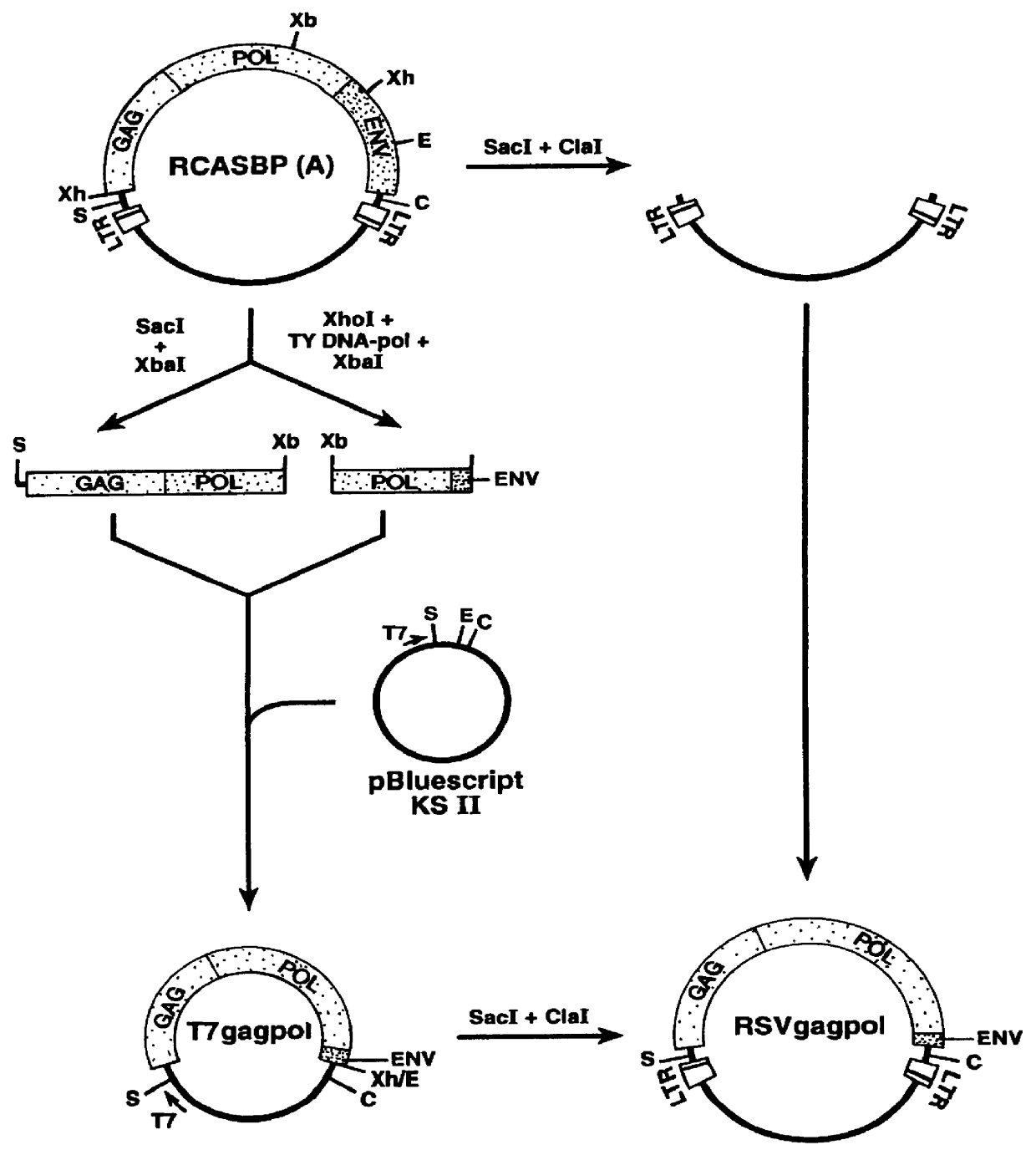
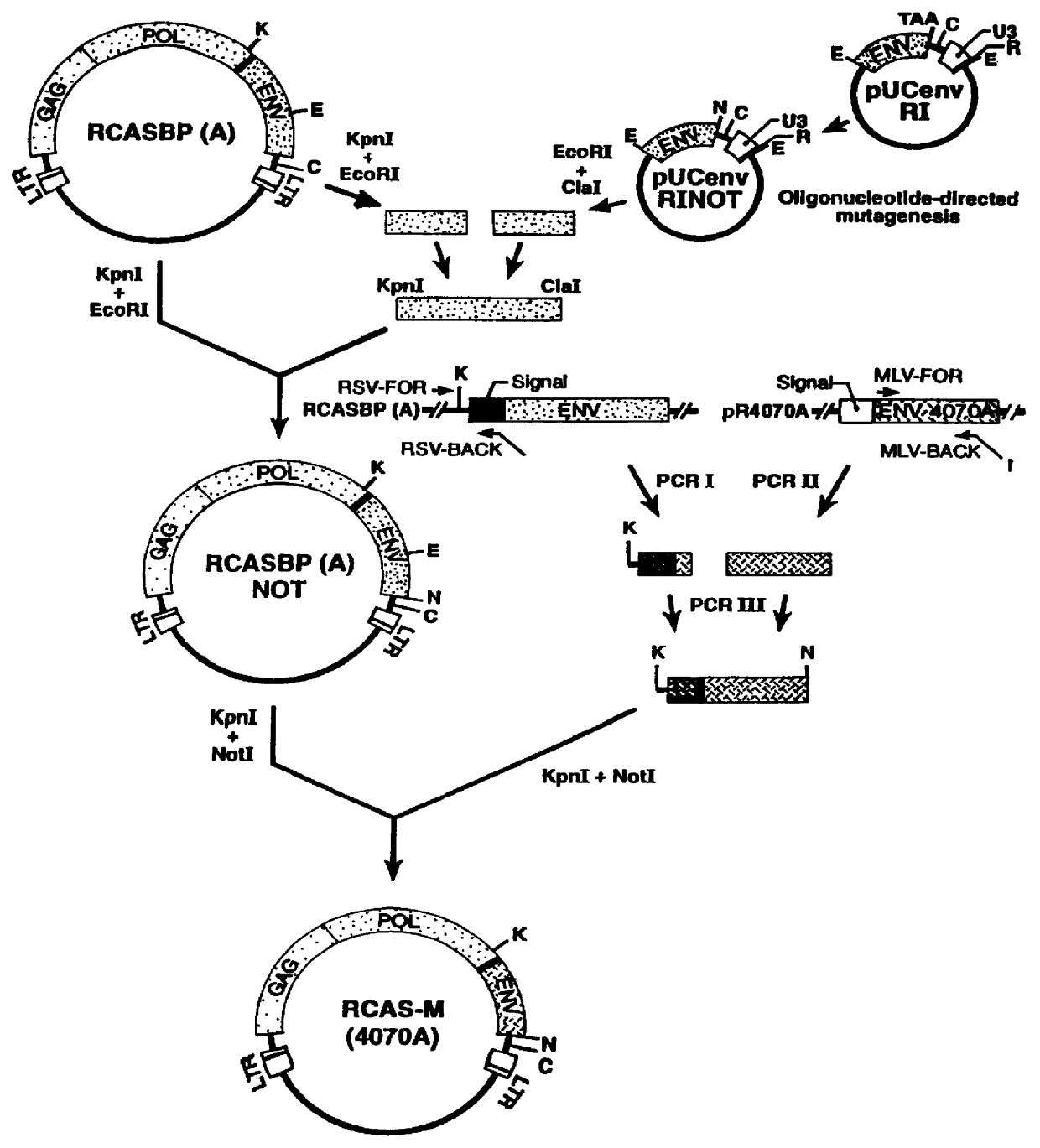
InactiveUS6096534AHigh titer stockEasy to usePolypeptide with localisation/targeting motifVectorsFowlAvian leukosis viruses
PCT No. PCT / US96 / 07370 Sec. 371 Date Nov. 28, 1997 Sec. 102(e) Date Nov. 28, 1997 PCT Filed May 22, 1996 PCT Pub. No. WO96 / 37625 PCT Pub. Date Nov. 28, 1996Recombinant avian sarcoma leukosis virus (ASLV)-derived retrovirus vectors having an expanded host range are described. The host range is expanded by the replacement of the ASLV envelope gene by an envelope gene from a virus capable of infecting both mammalian and avian cells. The resulting recombinant ASLV-derived retroviral vectors can replicate efficiently in avian cells, infect both avian and mammalian cells in high titer, and are replication-defective in mammalian cells. Thus, they are quite safe and advantageous for use in gene therapy and vaccines.
Owner:UNITED STATES OF AMERICA
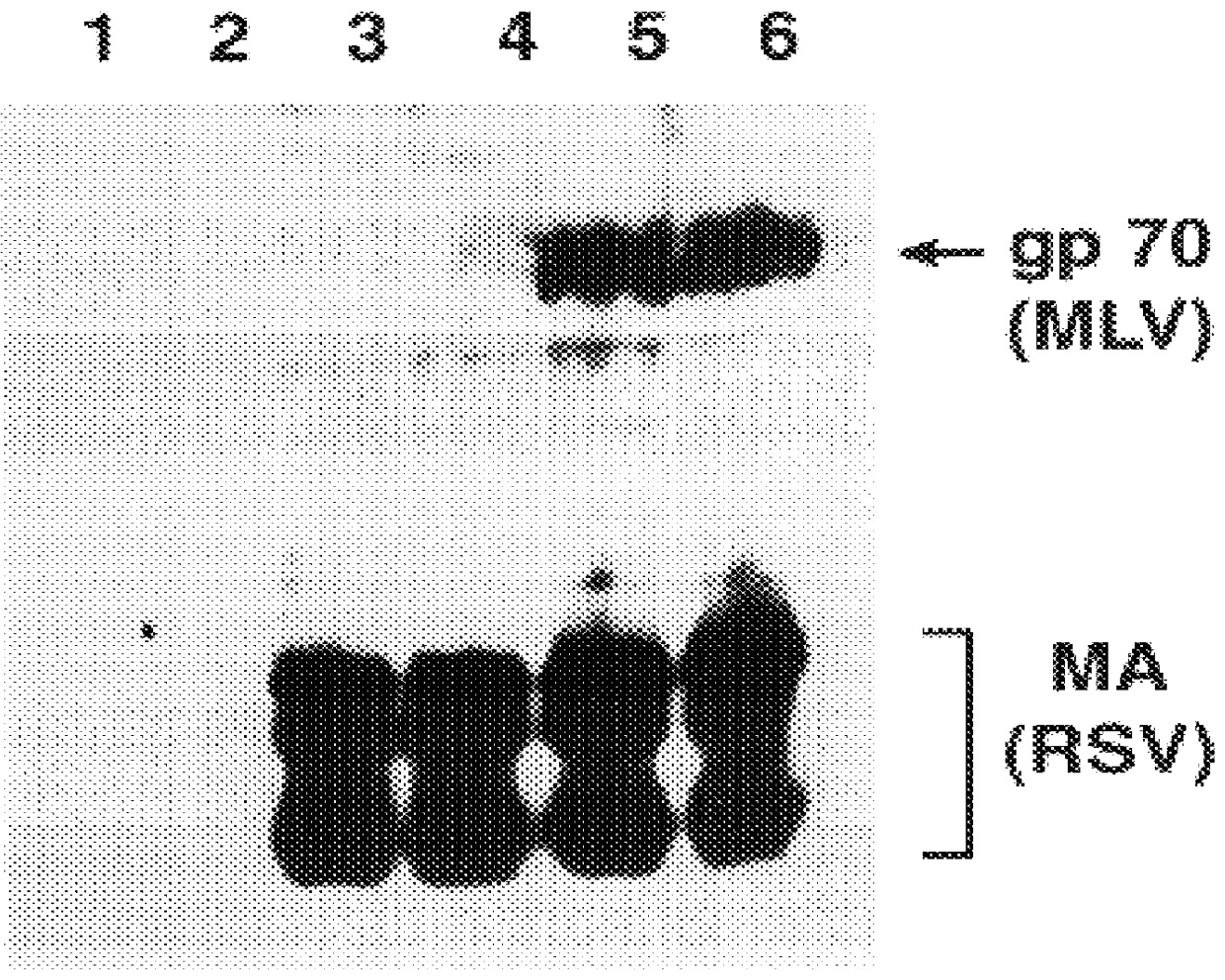
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Gene transfer composition and method
InactiveUS20050053910A1Improve efficiencyMinimisation of time and expenditureNew breed animal cellsGenetically modified cellsTransgeneCell culture media
Owner:REVIVICOR INC
Gene transfer for studying and treating a connective tissue of a mammalian host
Methods for introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule that contains the gene encoding for the product, and infecting the target cells of the mammalian host using the DNA vector molecule. A method to produce an animal model for the study of connective tissue pathology is also disclosed.
Owner:UNIVERSITY OF PITTSBURGH
Cotton breeding method
InactiveCN101707963ASimple Breeding MethodBroad Breeding PathwayPlant tissue cultureHorticulture methodsAgricultural scienceGossypium
The invention relates to a cotton breeding method. In the method, a combination part of genetic variation generated by cotton graft is used as an explant for inducing to culture callus; the cultured callus is subjected to propagation, subculturing and differentiated culture to obtain cotton seedlings; and after hardening off, the cotton seedlings are grafted for transplantation or directly transplanted. The cotton breeding method has the advantages that: by utilizing graft to create genetic variation, the method not only improves plant quality, yield, stress resistance, and the like, but also obtains distant variation which cannot be obtained through sexual hybridization, and has simple operation, high seed selection efficiency and low cost; the method provides a novel cotton transgenic means and can transfer target gene to a breed to be improved to generate a variation type combining the merits of parental stock and cion parents; and the creation of genetic variation by utilizing graft can be taken as a supplementary means of general breeding and modern gene engineering breeding and is an important breeding means with broad prospects.
Owner:HENAN ACAD OF AGRI SCI
Receptor-mediated gene transfer system for targeting tumor gene therapy
InactiveUS6339139B1Efficient targetingGrowth inhibitionOrganic active ingredientsPeptide/protein ingredientsMultiple Tumor Suppressor-1Oligopeptide
The invention relates to a gene transfer system binding to a growth factor receptor, comprising 4-element complex gene transfer system consisting of ligand oligopeptide / polycationic polypeptide / endosome release oligopeptide / exogenous DNA or 3-element complex consisting of ligand oligopeptide / polycationic polypeptide / exogenous DNA. The invention exemplifies E5, GE7, GV1 and GV2 systems and they can be targeted for the introduction of exogenous genes into malignant tumor cells or tumor vascular endotheliocytes. They are also able to highly inhibit the growth of tumor cells in animals while p15, p16 or p21WAF-1 was used as exogenous genes. The system according to the invention is a new introduction system in tumor gene therapy.
Owner:SHANGHAI INST OF ONCOLOGY
Tree-type high-molecular polyamide-amine compound and its preparing process and application
InactiveCN1379053ABiodegradableHigh efficiency and low toxicityVector-based foreign material introductionSodium methoxideMolecular materials
A tree-type polyamide-amine material series includes G1.5, G2.0, G2.5, G3.5...G8.0 three-type high-molecular materials. The above-mentioned half generation tree-type polyamid-amine material is prepared through Michael addition reaction of integer generation one on methyl acrylate under the catalysis of sodium methoxide. The above-mentioned integer generation tree-type polyamide-amine material is prepared through amidation reaction of half generation one on ethanediamine. Its advantages are biodegradability and low poison. It can be used as gene transfer carrier and release controlling carrierof medicine.
Owner:WUHAN UNIV
High efficiency gene transfer and expression in mammalian cells by a multiple transfection procedure of MAR sequences
ActiveUS8252917B2Increase productionImprove methodSugar derivativesOther foreign material introduction processesNucleotideTransfection
The present invention relates to purified and isolated DNA sequences having protein production increasing activity and more specifically to the use of matrix attachment regions (MARs) for increasing protein production activity in a eukaryotic cell. Also disclosed is a method for the identification of said active regions, in particular MAR nucleotide sequences, and the use of these characterized active MAR sequences in a new multiple transfection method.
Owner:SELEXIS SA
Multifunctional molecular complexes for gene transfer to cells
InactiveUS20050239204A1Restore activitySsRNA viruses negative-senseMicroencapsulation basedChemistryCompound s
A multifunctional molecular complex for the transfer of a nucleic acid composition to a target cell is provided. The complex is comprised of A) said nucleic acid composition and B) a transfer moiety comprising 1) one or more cationic polyamines bound to said nucleic acid compositions, 2) one or more endosome membrane disrupting components attached to at least one nitrogen of the polyamine and 3) one or more receptor specific binding components.
Owner:WYETH
Stable Gene Transfer to Proliferating Cells
InactiveUS20170216456A1Stable integrationStable expressionHydrolasesTransferasesDiseaseHepatic Diseases
Provided herein are methods for facilitating or inducing stable transgene integration and expression in a proliferating cell, comprising administering to the cell (i) a recombinant AAV (rAAV) vector comprising the transgene flanked by transposon-derived inverted terminal repeat sequences, which sequences are in turn flanked by AAV-derived inverted terminal repeat regions, and (ii) a source of a transposase that recognises said transposon-derived inverted terminal repeat sequences and directs the genomic integration of the transgene into the genome of the proliferating cell. Also provided are methods and transgene delivery systems for the treatment or prevention of diseases affecting, associated with or characterised by proliferating cells, including paediatric liver diseases, bone marrow diseases and cancer.
Owner:MOUNT SINAI HOSPITAL +2
Genetically modified T-cells, method for producing them and use thereof
InactiveUS20020119571A1Prevent rejectionBiocideGenetic material ingredientsT cellDrainage lymph nodes
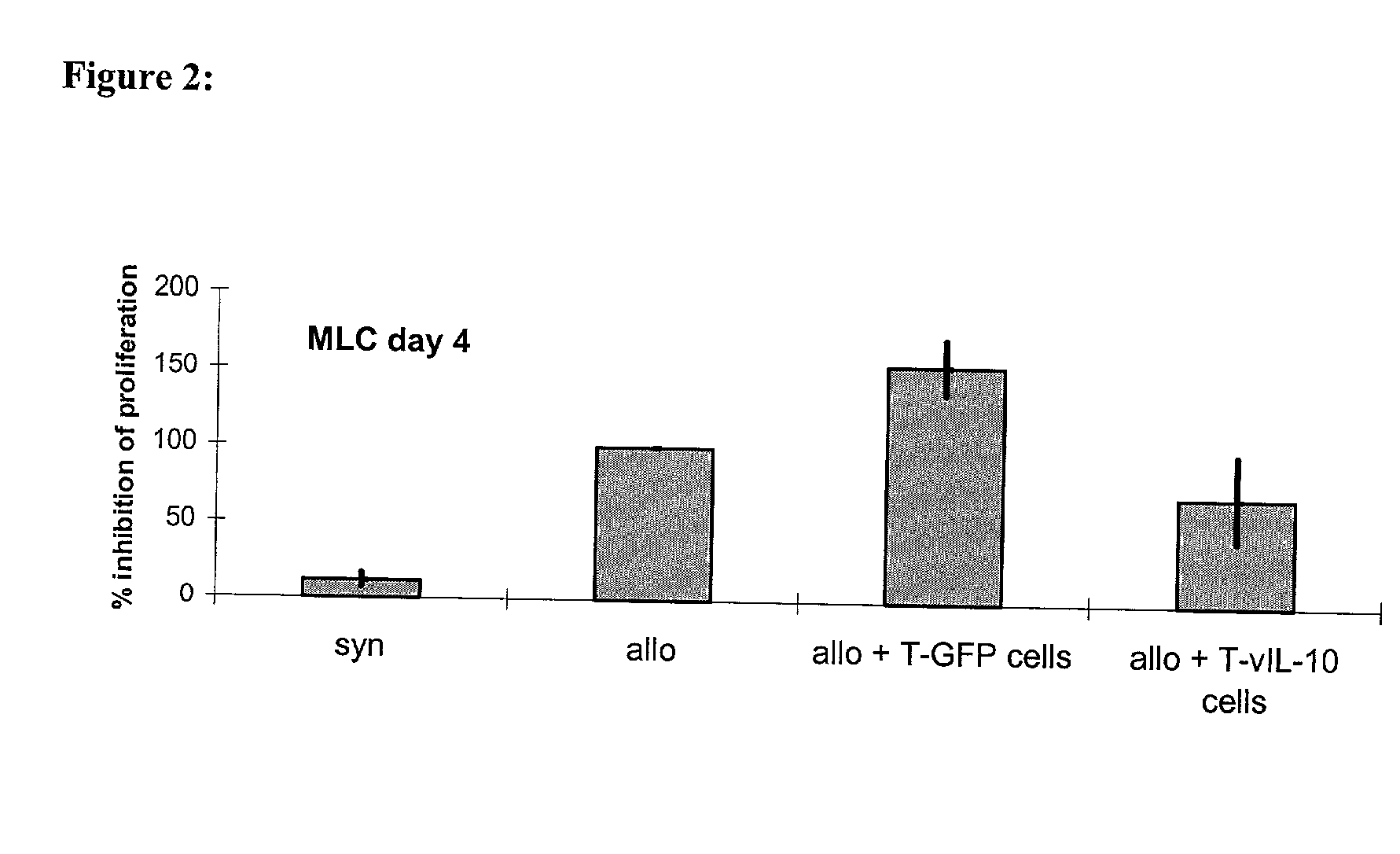
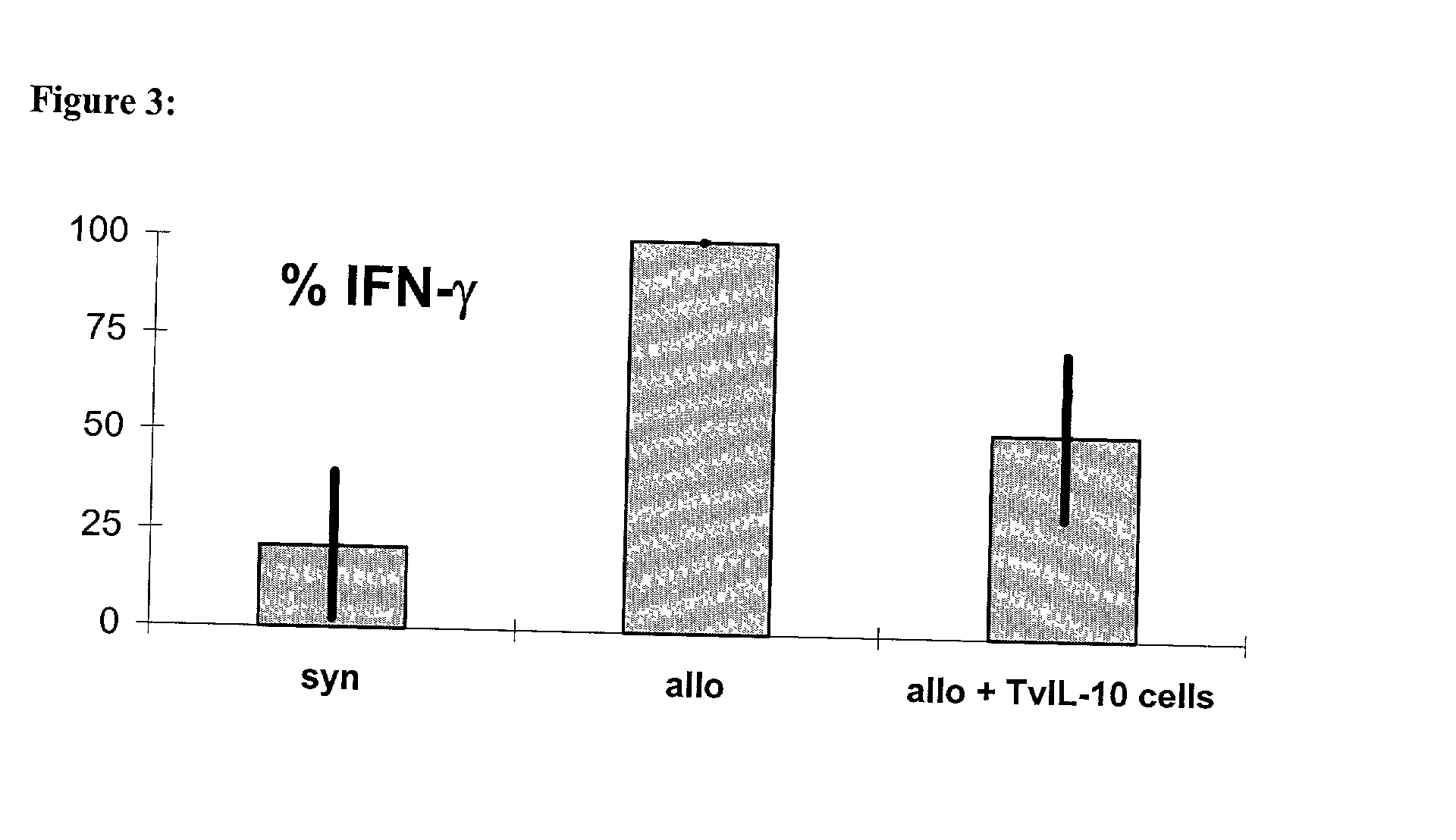
T cells of a graft recipient are stimulated in-vitro by cells of a graft donor or by cells which express dominant MHC-molecules, while they are transduced with immuno-modulatory genes using gene transfer. Following the gene transfer the transduced T cells start to express immuno-modulatory genes. The gene transfer can be accomplished using retroviruses, other viral vector systems or liposomes. Due to the chosen set-up of the experiment, which leads to the generation and expansion of allospecific T cells, the T cells migrate, after the in-vivo application, specifically into the allogeneic graft as well as into the draining lymph nodes and are then able to express the immuno-modulatory genes there. Rejection of allogeneic grafts (cells, tissues, organs) can be successfully prevented, tolerance towards allogeneic grafts can be induced and maintained.
Owner:UNIVSKLINIKUM CHARITE MEDIZINISCHE FAKULTAET DER HUMBOLDT UNIV ZU BERLIN
Electroporation method including the use of depressurization/pressurization
InactiveUS20060188992A1Efficient transferEasy to introduceBioreactor/fermenter combinationsBiological substance pretreatmentsElectricityGene transfer
An object of the present invention is to establish a transformation method simpler and more rapid than conventional techniques. This object can be solved by a method comprising the steps of a) holding a cell under a pressure different from an atmospheric pressure, and b) placing the cell and a nucleic acid under conditions capable of inducing electroporation. The present invention removes the necessity of culture which is conventionally required after gene transfer. Therefore, the present invention makes it possible to obtain a transformant of species which are impossible or considerably difficult to transform by conventional techniques. The simple transformation method of the present invention easily allows large-scale processing and large-scale analysis for research and development in the art. In addition, the present invention triggers dramatic advances in research, potentially leading to the development of innovative recombinants.
Owner:NAT INST OF AGROBIOLOGICAL SCI
Peptide-mediated gene transfer
InactiveUSRE39220E1Improve efficiencyProlong lifePeptide/protein ingredientsPeptide sourcesHinge regionPrimary cell
A methodology that allows for highly efficient transfer and stable integration of DNA into both established eukaryotic cell lines and primary cells, including non-dividing cells such as human peripheral blood monocytes and macrophages, entails the use of a synthetic polypeptide comprised of a peptide domain which corresponds to a nuclear localization signal sequence and a DNA binding domain which is rich in basic amino acids, separated by a hinge region of neutral acid which prevents stearic interference between the two domains.A synthetic polypeptide that allows for highly efficient transfer of DNA into eukaryotic cells, including, for example, non-dividing cells such as human peripheral blood monocytes and macrophages.<?insert-end id="INS-S-00001" ?>
Owner:GENETIC APPL
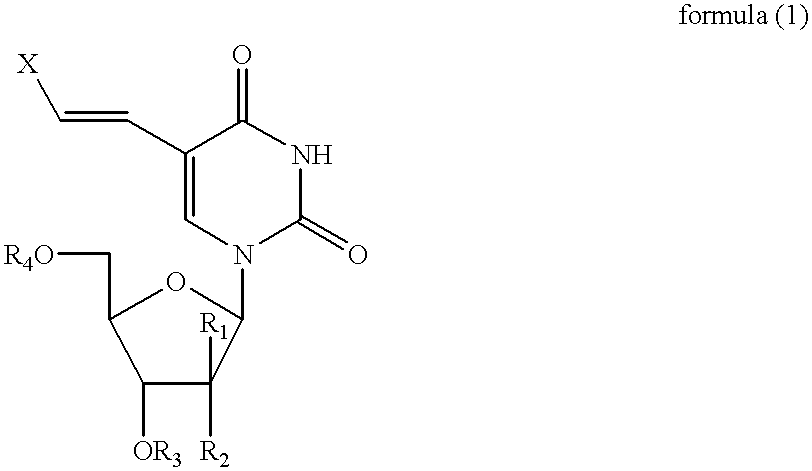
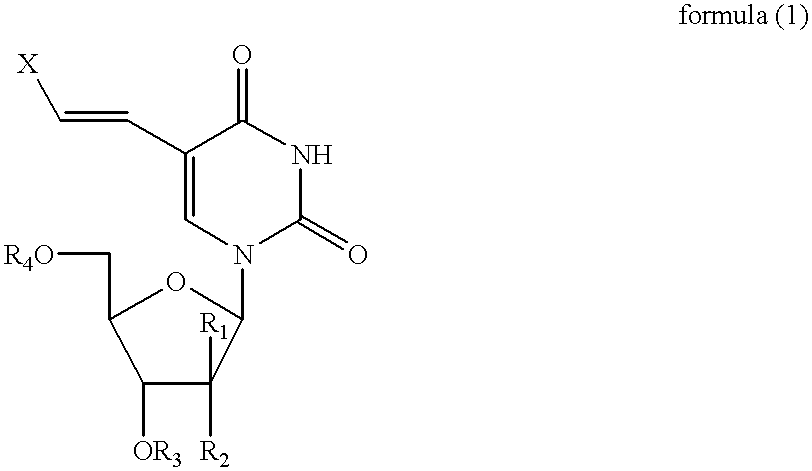
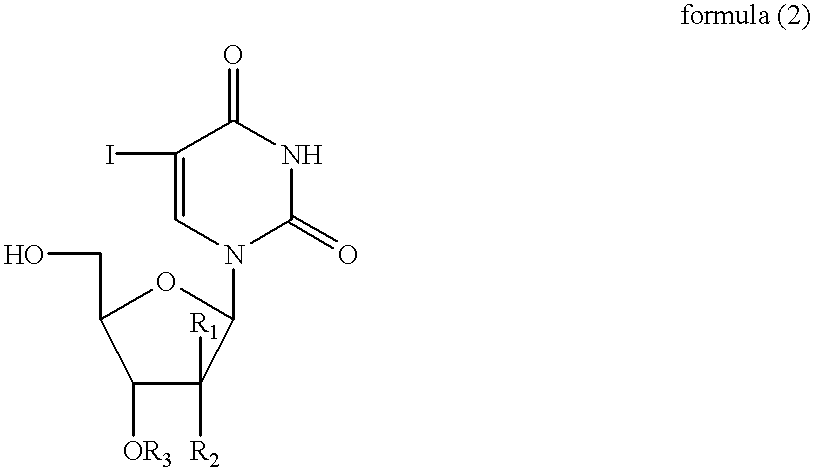
Combined use of nucleoside analogues and gene transfection for tissue imaging and therapy
InactiveUS20020025296A1Easily expelledEasy to detectMicrobiological testing/measurementGenetic material ingredientsCombined useFhit gene
A method and use of a labelled compound for monitoring the transfer of a foreign gene including selecting the foreign gene which has been isolated from a cell or virus and transferred into a cell population and selecting the labelled compound which will interact selectively with a protein expressed by the foreign gene to produce a labelled product. The labelled compound has a rate of expulsion from the cells which is greater than that of the labelled product. Further, the use and method include administering to the cells an effective dose of the labelled compound such that the labelled compound selectively interacts with the protein to produce the labelled product, waiting a period of time such that a substantial amount of the labelled compound has been expelled from the cells and such that a detectable amount of the labelled product remains and determining the extent and location of the protein by detecting the labelled product.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com