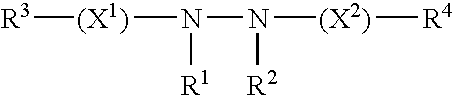Methods for modulating expression of exogenous genes in mammalian systems, and products related thereto
a technology of exogenous genes and products, applied in the field of recombinant dna technology, can solve the problems of slow clearance of antibiotics from bone, tetracycline pharmacokinetics may hinder its use during development, etc., and achieves convenient storage, rapid clearance, and precise and potent induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Modified Ecdysone Receptors
Plasmid Preparation:
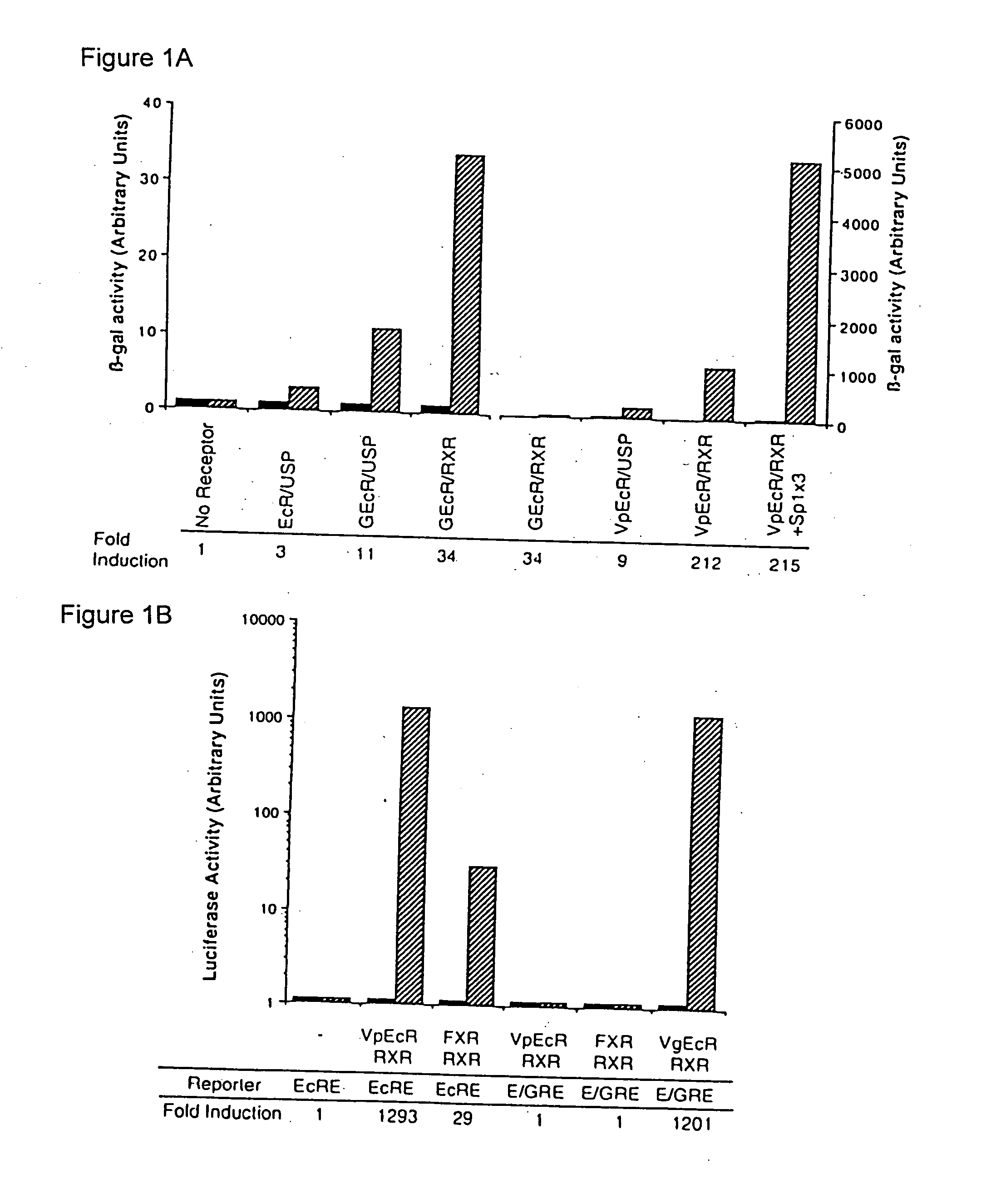
[0176] The plasmids CMX-EcR, CMX-USP, CMX-FXR, CMX-hRXRa, EcREx5-ΔMTV-Luc, CMX-GEcR, MMTV-luc, and CMX-GR have been previously described (Yao, et al., Nature 366:476-479 (1993) and Forman, et al. Cell 81:687-693 (1995)).
[0177] The plasmid CMX-VpEcR was constructed by ligation of an EcoRI fragment of psk-EcR and CMX-Vp16.
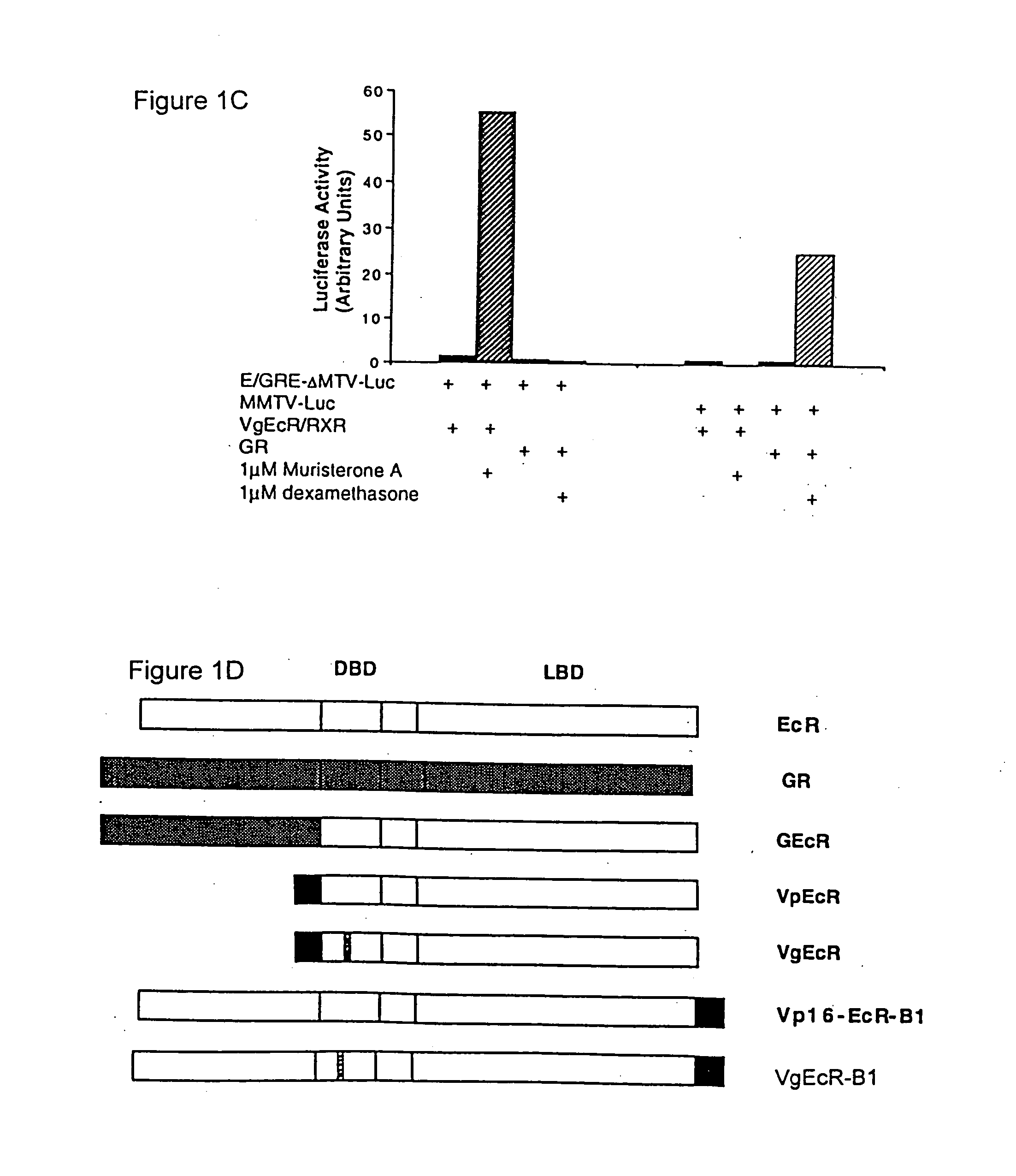
[0178] The plasmid CMX-VgEcR was generated by site-directed mutagenesis of CMX-VpEcR using the Transformer Mutagenesis Kit (Clontech) and the mutagenic oligonucleotide (SEQ ID NO:14): [0179] 5′-TACAACGCCCTCACCTGTGGATCCTGCAAGGTGTTTCTTTCGACGCAGC-3′.
Mutagenesis of VpEcR to VgEcR altered the P-box region of the DNA binding domain of ecdysone receptor to resemble that of GR (Umesono and Evans, Cell 57:1139-1146 (1989)). The following amino acids in the DNA-binding domain of the ecdysone receptor were altered: E282G, G283S, and G286V (E=glutamate, G=glycine, S=serine, V=valine).
[0180] The reporter constr...
example 2
Construction of a Novel Ecdysone Response Element
[0192] Although no mammalian transcription factors have been shown to have a natural enhancer element like the ecdysone response element, which is composed of two inverted half-sites of the sequence AGGTCA spaced by one nucleotide, it is difficult to preclude such a possibility. The recently cloned farnesoid X receptor (FXR) can very weakly activate certain synthetic promoters containing an ecdysone response element in response to extremely high concentrations of farnesoids (Forman et al., Cell 81:687-693 (1995)).
[0193] In FXR containing cells and in transgenic mice, activation of gene expression by endogenous receptors would create undesirable background levels of reporter protein. To circumvent this potential problem, the DNA binding specificity of VpEcR was altered to mimic that of GR, which binds as a homodimer to an inverted repeat of the sequence AGAACA, spaced by three nucleotides. This altered binding specificity was achieve...
example 3
Assay for Ecdysone Responsiveness in Stable Cell Lines
[0194] Stable cell lines were generated containing the modified ecdysone receptor VpEcR, a heterodimeric partner (RXR), and an ecdysone inducible reporter (FIG. 2). 293 cells were transfected with the following linearized plasmids, pRC-ESHβ, EcREx5-ΔMTV-Luc, CMX-VpEcR, and CMX-hRXRa. The following day, the cells were split 1:10 and were allowed to recover one day prior to selection with 1 mg / ml G418 (GIBCO). After 14 days of selection, 14 individual clones were isolated and grown separately in the presence of 0.5 mg / ml G418. Of 14 G418 resistant clones, 10 demonstrated differing degrees of muristerone responsiveness.
[0195] One of these cell lines, N13, was grown in the presence or absence of 1 μM muristerone for 20 hours. Cell lysates were then assayed for β-galactosidase and luciferase activities as described in Example 1. X-gal staining was performed on the stable cell lines. Cells were fixed briefly with 10% formaldehyde in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com