Vaccine composition, and preparation method and application thereof
A technology of vaccine composition and Muscovy duck parvovirus, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems such as the impact of the immune effect of the attenuated live vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
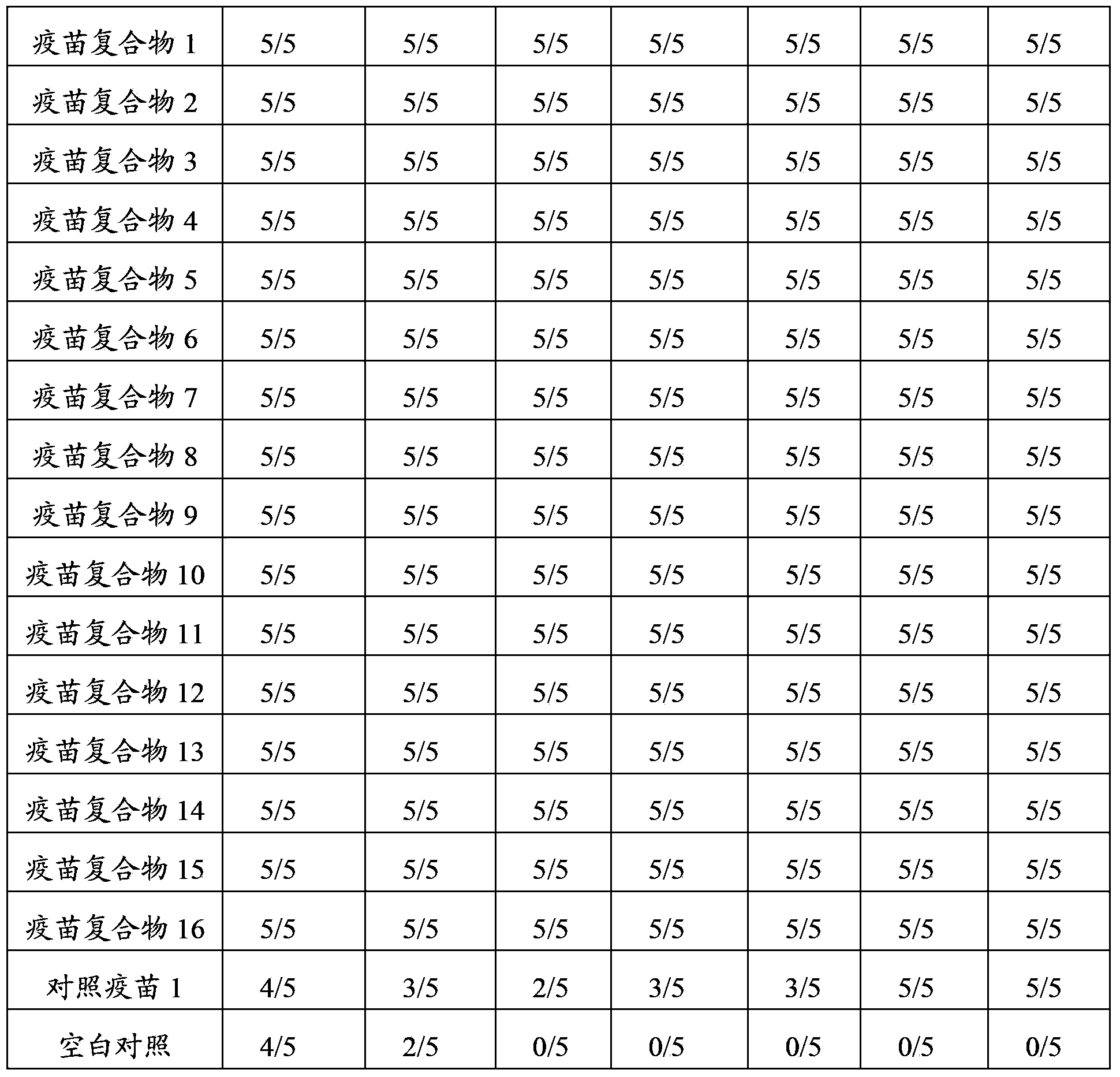
[0036] Example 1 Preparation and Application of Muscovy Duck Parvovirus M91-G27 Strain Antigen-Antibody Complex Vaccine
[0037] 1.1 Preparation of Muscovy duck parvovirus M91-G27 strain antigen
[0038] 1.1.1 Preparation of whole virus antigen of Muscovy duck parvovirus M91-G27 strain
[0039]Take the Muscovy duck parvovirus M91 strain and use the published method (Wang Yongkun, Qian Zhong, Qian Shumei, etc. Comparison of the characteristics of duckling duck parvovirus and gosling plague virus and the application of dual live vaccines[J], China Poultry Industry Guide, 2004, 21 (9): 18-20), first passed 3 generations in muscovy duck embryos, named M91 strain, and then continuously passed 27 generations in 12-day-old goose embryos, named M91-G27 strain. The M91-G27 strain was inoculated with goose embryos for propagation, and the infected goose embryo liquid was harvested to prepare the whole virus antigen of Muscovy duck parvovirus M91-G27 strain. The viral content of the wh...
Embodiment 2
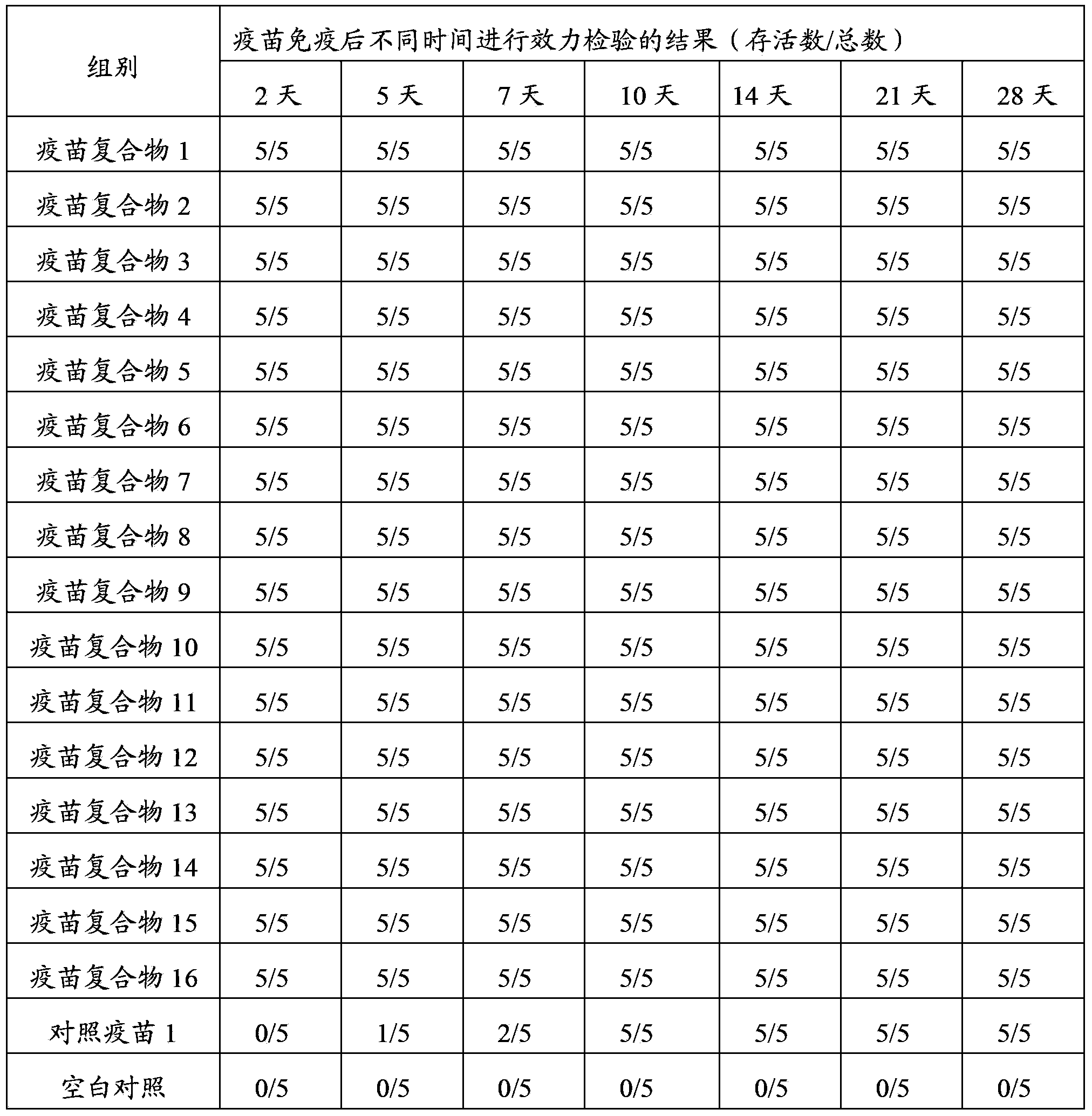
[0099] Example 2 Preparation and Application of Muscovy Duck Parvovirus P1 Strain Antigen-Antibody Complex Vaccine
[0100] 2.1 Preparation of Muscovy duck parvovirus P1 strain antigen
[0101] 2.1.1 Preparation of whole virus antigen of Muscovy duck parvovirus P1 strain
[0102] Take Muscovy duck parvovirus P1 strain seed virus, using the published method (Fujian Academy of Agricultural Sciences Institute of Animal Husbandry and Veterinary Medicine, Preparation method of new Muscovy duck parvovirus vaccine: China, 101880651A[P], 2010.11.10), seed the virus according to Muscovy duck embryo fibroblasts were inoculated synchronously with 1% of the culture solution, rotated at 37°C until the cytopathic rate reached more than 75%, and the cytotoxicity was harvested to make the whole virus antigen of Muscovy duck parvovirus P1 strain. The viral content of the whole virus antigen was determined to be 10 6.0 ELD 50 / 0.2ml.
[0103] 2.1.2 Eukaryotic expression of VP2 protein of Mu...
Embodiment 3
[0163] The preparation of embodiment 3 muscovy duck parvovirus Q strain antigen-antibody complex vaccine
[0164] 3.1 Preparation of Muscovy Duck Parvovirus Q Strain Antigen
[0165] 3.1.1 Preparation of whole virus antigen of Muscovy duck parvovirus Q strain
[0166] Take Muscovy duck parvovirus Q strain seed virus, using the published method (Lou Hua, Wang Zhengfu, Xu Bin. Development and application of Muscovy duck parvovirus attenuated vaccine [J], China Veterinary Science and Technology, 1998, 28(3): 24-25), the virus was properly diluted with sterilized saline, inoculated with muscovy duck embryos for continuous passage, when the virus content reached 10 5 ELD 50 When it is more than 0.2ml, it can be used as the whole virus antigen of the Q strain of parvovirus. The viral content of the whole virus antigen was determined to be 10 6.4 ELD 50 / 0.2ml.
[0167] 3.1.2 Eukaryotic expression of Muscovy duck parvovirus Q strain VP2 protein
[0168] Infected duck embryo fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com