Infectious bursal disease live vaccine and production method thereof
A chicken infectious and bursal disease technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of antigenic variation of strains in the field, economic loss, immune failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
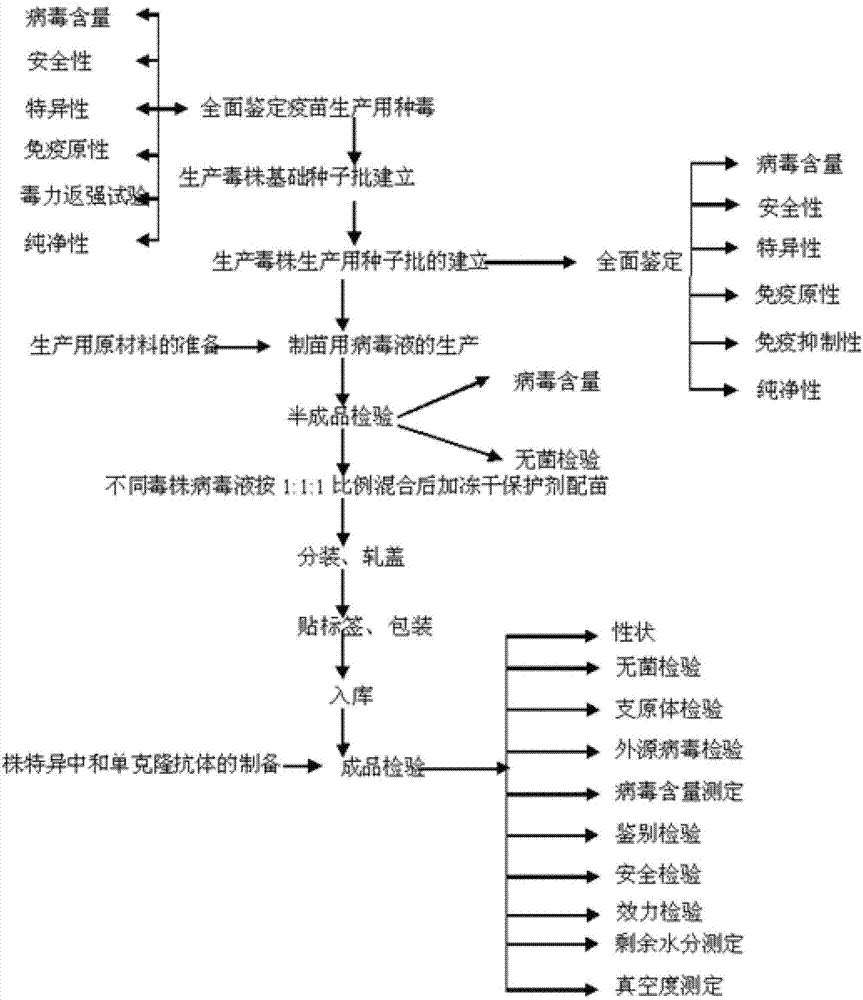
Image
Examples
Embodiment 1
[0082] ——— Vaccine Manufacturing
[0083] 1. Materials
[0084] (1) Virus content of chicken infectious bursal disease virus B87 strain 10 6.38 ELD 50 / 0.2ml, provided by Jilin Zhengye Biological Products Co., Ltd.
[0085] (2) Virus content of chicken infectious bursal disease virus CA-C strain 10 6.5 ELD 50 / 0.2ml, provided by Beijing Haidian Zhonghai Animal Health Technology Company.
[0086] (3) Virus content of chicken infectious bursal disease virus CF-C strain 10 6.5 ELD 50 / 0.2ml, provided by Beijing Haidian Zhonghai Animal Health Technology Co., Ltd.
[0087] (4) The virulent chicken infectious bursal disease BC6-85 virulent strain used for inspection was provided by the China Veterinary Drug Control Institute.
[0088] (5) SPF chicken embryos were purchased from Guangdong Dahuanong and Beijing Lanfeng Poultry Breeding Co., Ltd.
[0089] 2. Seed poison preparation for production
[0090] (1) Chicken infectious bursal disease virus B87 strain
[0091] 1) Pr...
Embodiment 2
[0129] ——— Finished product inspection (the vaccine prepared by the embodiment, the batch number is 01 batches)
[0130] 1. Properties The vaccine is a light red spongy loose mass, which is easy to detach from the bottle wall and dissolves quickly after adding diluent.
[0131] 2. The sterility test of the vaccine is carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and there is no growth of bacteria and mold.
[0132] 3. The mycoplasma test vaccine was carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and there was no mycoplasma growth.
[0133] 4. Exogenous virus inspection The vaccine is tested according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and there is no exogenous virus contamination.
[0134] 5. Differential test Dilute the vaccine to 1 part / 0.1ml, mix it with the same amount of specific anti-infectious bursal virus serum, neutralize at 37°C for 60 minutes, a...
Embodiment 3
[0144] ----Clinical Trials
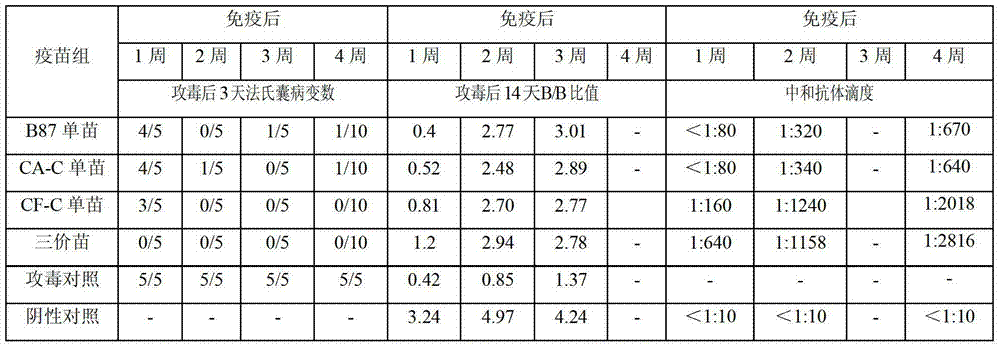
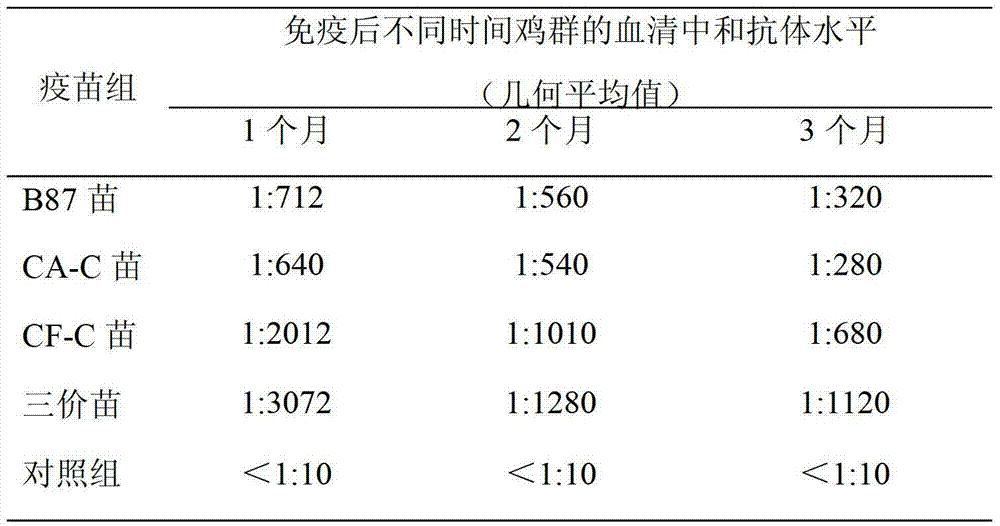
[0145] The present inventor has carried out clinical trials with 3 batches of pilot-scale products of chicken infectious bursal disease trivalent live vaccine (B87 strain + CA-C strain + CF-C strain). More than 16,000 Nongda No. 3 laying hens and 18,000 Ross 308 broiler chickens were immunized in the experiment. After the test vaccine was inoculated with layer hens and broiler chickens at the recommended dose and overdose (10 times the recommended dose), no adverse reactions caused by the vaccine were observed. 14 days after overdose inoculation, randomly select laying hens and broilers for necropsy, observe the bursa, liver, kidney, spleen, glandular stomach, gizzard, chest, leg and wing muscles, except for the mild atrophy of the bursa , other organs and muscles had no visible pathological changes caused by chicken infectious bursal disease. 14 days after immunization of broiler and laying hens with dose and 1 / 2 dose, challenge with virulent B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com