Chimeric Newcastle disease virus vaccine vector candidate strain capable of overcoming influence of maternal antibody of Newcastle disease and construction method thereof
A technology of Newcastle disease virus and maternal antibody is applied in the field of developing Newcastle disease vector vaccine, which can solve the problems of changing antigenicity and reducing virus replication efficiency, and achieve the effect of broad application prospect and high reproduction performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
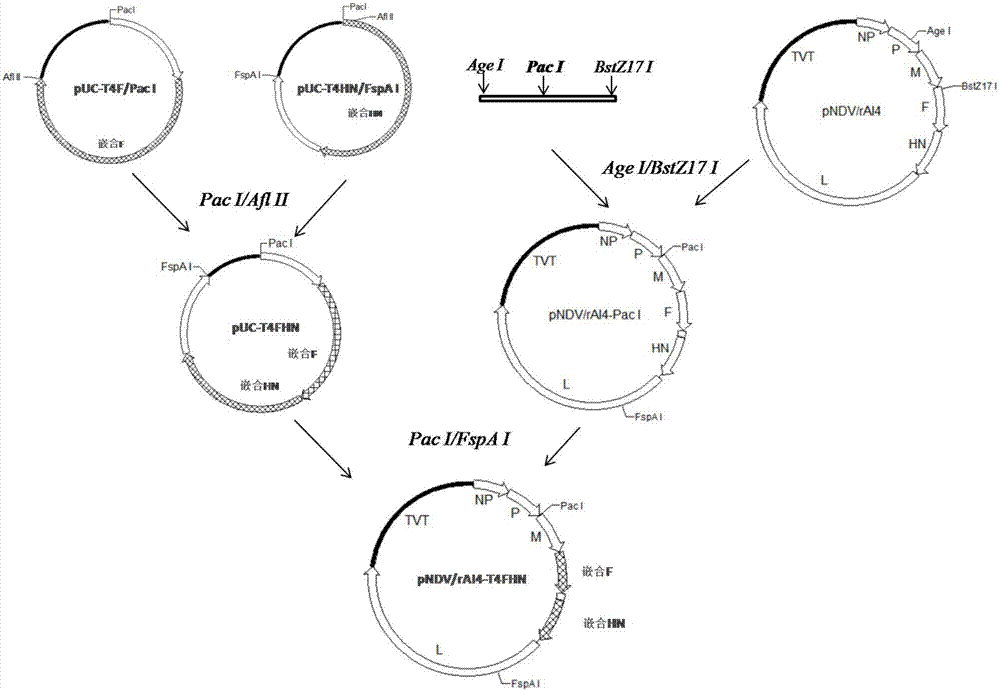
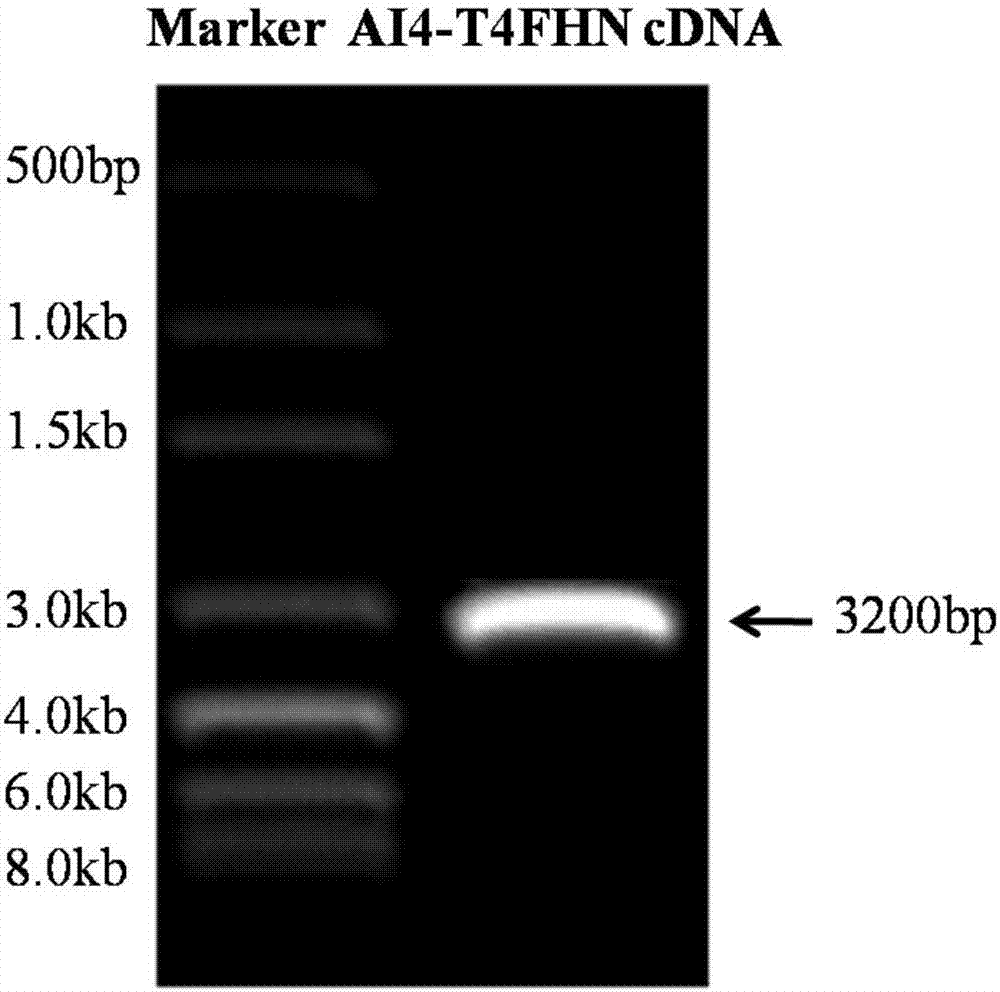
[0031] The construction model of recombinant plasmid pNDV / rAI4-T4FHN is as follows figure 1 .
[0032] Construction Step 1: Modification of Newcastle Disease Virus AI4 Genome
[0033] Biomaterial preparation:
[0034] The full-length expression vector pNDV / rAI4 of NDV AI4 strain (Hu Z, Hu S, Meng C, et al. Generation of a Genotype VII Newcastle Disease Virus Vaccine Candidate with High Yield in Embryonated Chicken Eggs. Avian Diseases 2011,55(3):1759- 1769) was constructed and preserved by the Key Open Laboratory of Livestock and Poultry Infectious Diseases of the Ministry of Agriculture of Yangzhou University. pCR2.1 vector: purchased from Invitrogen; AMV reverse transcriptase, High fidelity DNA polymerase, T4 DNA ligase, and Agarose Gel DNA Extraction Kit were purchased from Roche; transfection reagent SuperFect and plasmid extraction kit (QIAprep Spin MiniPrep Kit) It is the product of QIAGEN; the rest of the conventional reagents are domestic analytical grade.
[0035]...
Embodiment 2
[0073] Embodiment 2: the biological characteristic identification of recombinant virus
[0074] 1. Determination of MDT and ICPI
[0075] Chicken embryo mean death time (mean death time, MDT) measurement: Recombinant virus rAI4-gB was made 10 times (10 times) respectively with sterilized physiological saline. -6 、10 -7 ...10 -10 ) gradient dilution, each dilution was inoculated with 5 9-11-day-old SPF chicken embryos, 0.1 mL per embryo, and 5 chicken embryos inoculated with normal saline were set as a control. Incubate at 37°C, discard chicken embryos that died within 24 hours, then observe every 12 hours until 120 hours, and calculate the MDT of the virus according to the OIE standard method. Results: Chicken embryos inoculated with 5 dilutions of the virus did not die within 120 hours, and the MDT value of this strain was greater than 120 hours.
[0076] Determination of intracerebral pathogenicity index (ICPI) inoculation in chicks: take fresh virus allantoic fluid with...
Embodiment 3
[0079] Embodiment 3: Immunological potency test of vaccine carrier strain
[0080] The 1-day-old commercial chickens and SPF chickens were randomly divided into 10 chickens / group, and kept in isolation, and were inoculated with the vaccine vector strain AI4-T4FHN and LaSota attenuated vaccine respectively through nasal drops and eye drops, for 10 days. 6 EID 50 / only, and the PBS inoculated group was set as the control. Venous blood was collected from each test chicken 2 weeks after immunization, and the level of seroconversion against the immune strain was detected. The result is as image 3 , AI4-T4FHN and LaSota immunized chickens did not show any clinical symptoms, indicating that the AI4-T4FHN vaccine vector strain is not pathogenic to chickens and can be used safely. LaSota attenuated vaccine immunization of 1-day-old commercial chickens is greatly affected by maternal antibodies, which cannot effectively stimulate antibody production and can only maintain serum titer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com