Application of chitosan in avian vaccine composition preparation
A technology of vaccine composition and chitosan solution, which is applied in the application field of poultry vaccine composition, can solve the problems of increasing vaccine cost, increasing animal stress and drug residue, and achieving improved resistance, less antigenicity, The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
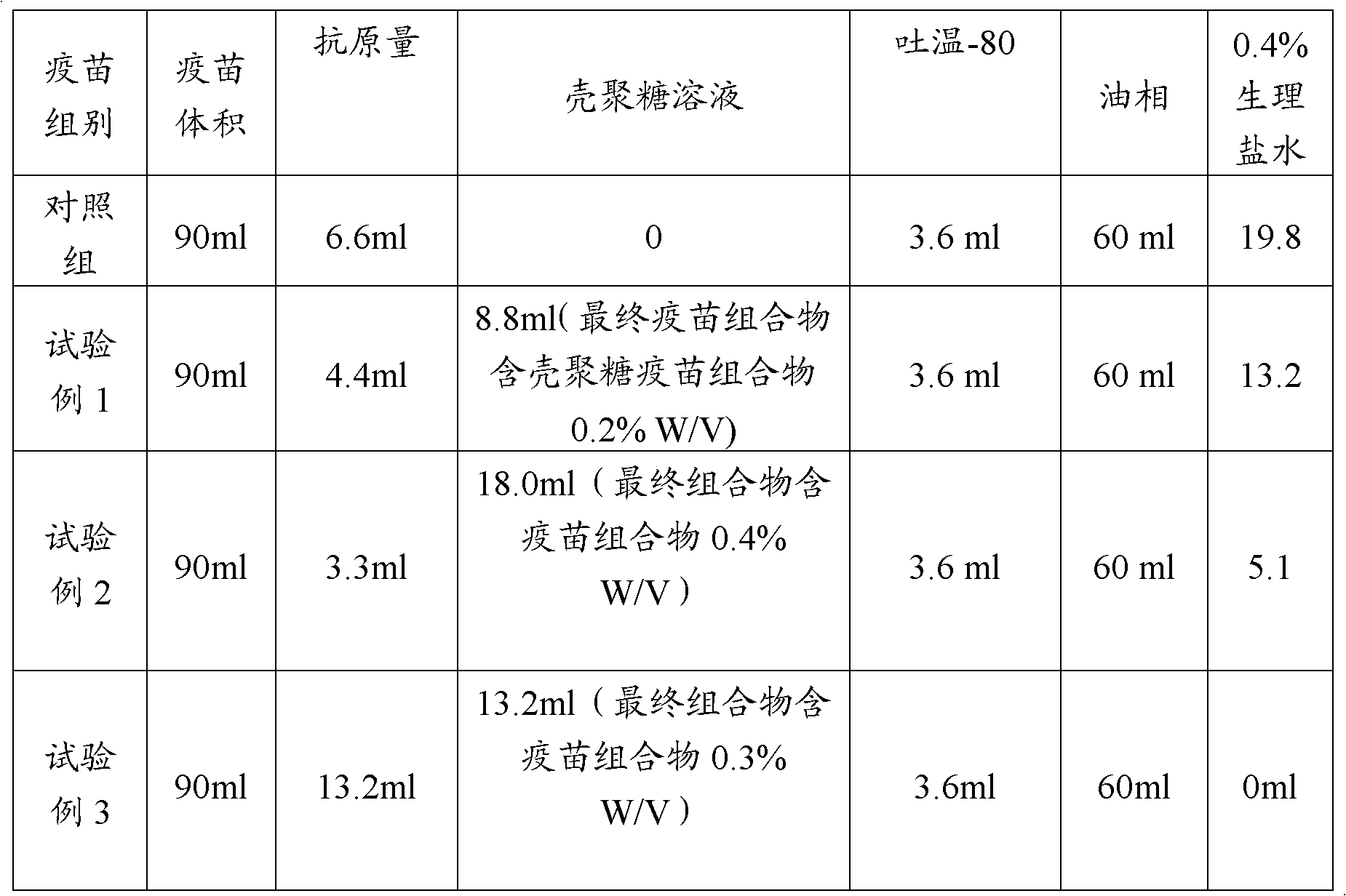
[0020] Embodiment 1: the preparation that contains the chitosan solution of 2%W / V:
[0021] Dissolve 4.1 g of sodium acetate (purchased from Sigma Chemical Co., St. Louis, MO) in 50 ml of deionized water for 0.5 M sodium acetate solution, adjust the pH to 4.5 with about 7 ml of glacial acetic acid, and then add 1.5 ml of glacial acetic acid to compensate for polymerization. The effect of the addition of glucosamine on the pH of the solution. The total volume of the solution was adjusted to 100ml with deionized water.
[0022] 2g of chitosan (product of Shanghai Qisheng Biological Products Co., Ltd., degree of polymerization 88.5%) was slowly added to the above sodium acetate solution and stirred continuously for 2-3 hours until the chitosan was dissolved. Filter through a 0.22 μm membrane to remove impurities, autoclave at 112°C for 30 minutes, and store at 4°C.
Embodiment 2
[0023] Embodiment 2: the application of chitosan in the adjuvant of preparation Newcastle disease vaccine composition
[0024] 1. Newcastle disease virus (hereinafter referred to as NDV) Lasota strain propagation and identification
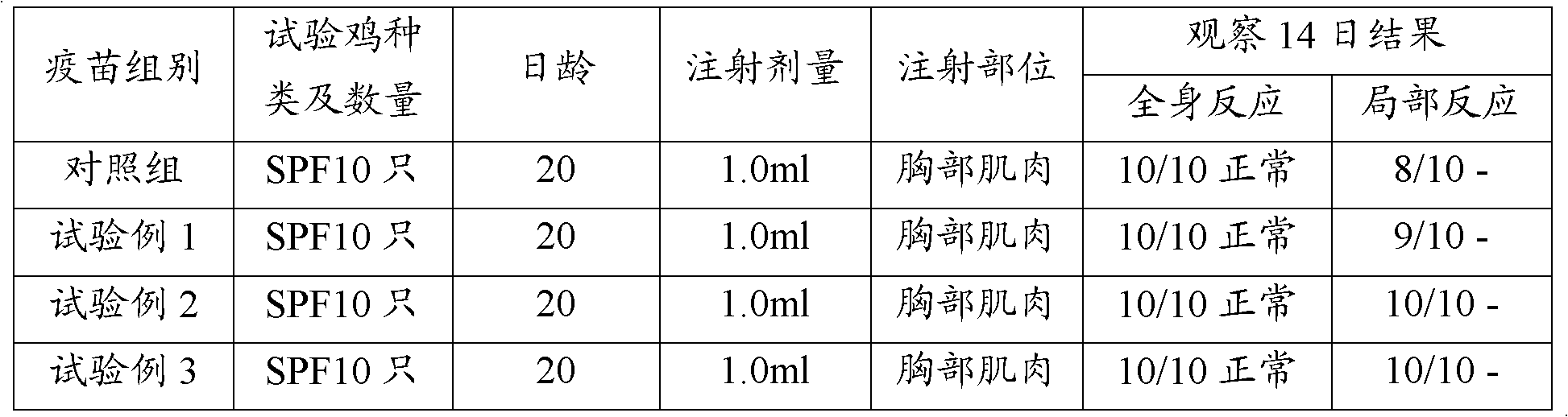
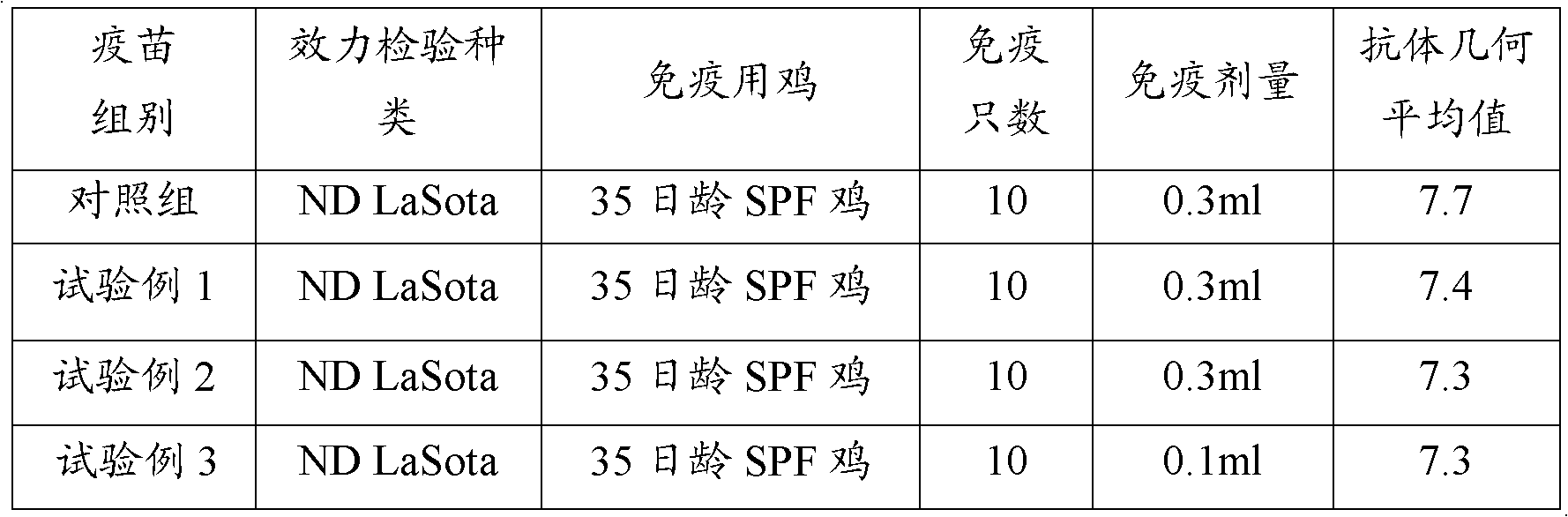
[0025] 1.1 Newcastle disease virus Lasota strain of breeding chickens was purchased from China Veterinary Drug Control Institute. Properly dilute the virus species with sterilized physiological saline (10 -3 ~10 -4 ), inoculate 10-day-old SPF embryos at 0.1ml per allantoic cavity, culture them for 120 hours, photograph the embryos once every 4-6 hours, take out the dead embryos in time, and select the ones that died 72-120 hours after inoculation and had obvious lesions. As for the chicken embryo, the chicken embryo liquid is harvested separately and put into a sterilized container. The chick embryo fluid tested for sterility and HA ≥ 1:512 was mixed, quantitatively dispensed, 2ml per bottle, and stored in a freezer.
[0026] 1.2 Determination...
Embodiment 3
[0050] Embodiment 3, the application of chitosan in the adjuvant of preparation bird flu vaccine composition
[0051] 1. Propagation and identification of H9HL strain virus species
[0052] 1.1 Breeding of virus seeds: Avian influenza virus A / Chicken / Henan / Luoyang / HL / 2001 (H9N2) (referred to as HL strain). The HL strain freeze-dried virus was properly diluted with sterilized physiological saline (10 -3 ~10 -4 ), 0.1ml per allantoic cavity was inoculated with 10-day-old SPF embryos, and incubated at 37°C for 96 hours. During this period, the dead embryos before 48 hours were discarded. After 48 hours, the embryos were photographed once every 4 to 6 hours, and the dead embryos were removed in time. After 96 hours, take them all out, put them in the refrigerator at 2-8°C for more than 12 hours, harvest the chicken embryo fluids separately, put them into sterilized containers, mix the chicken embryo fluids that have been tested for sterility and have a HA≥1:512, and pack them qu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com