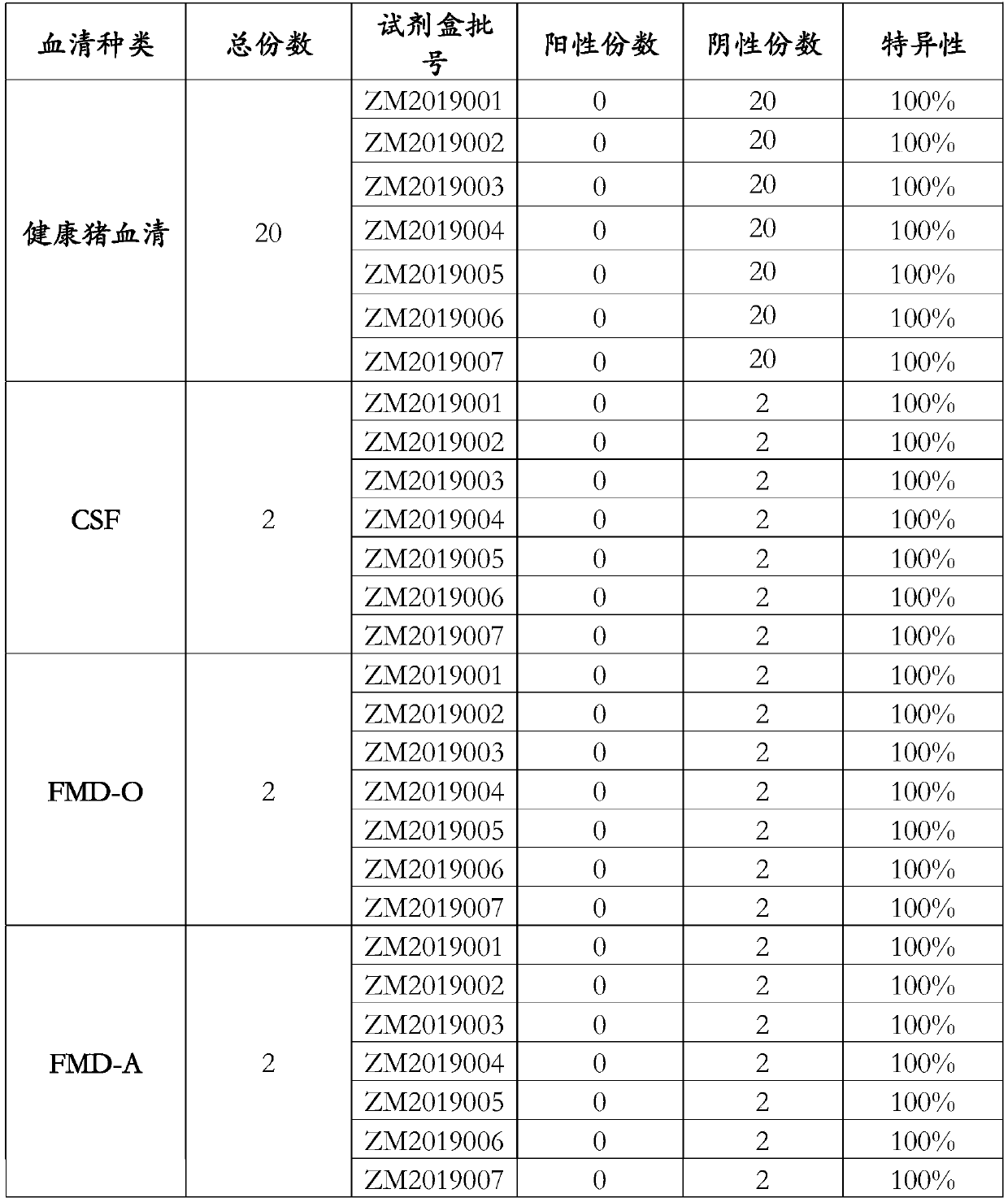
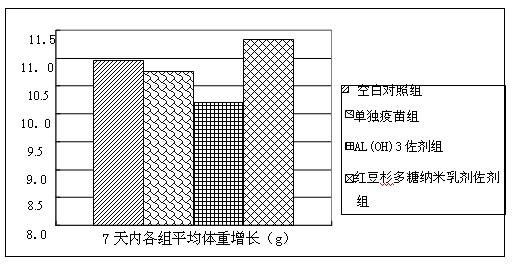
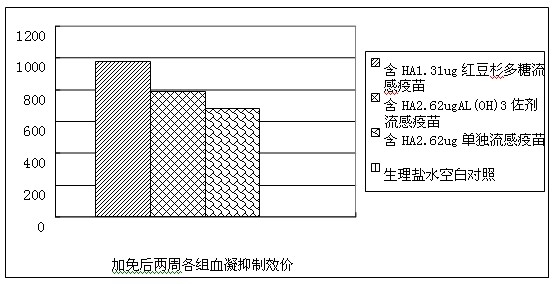
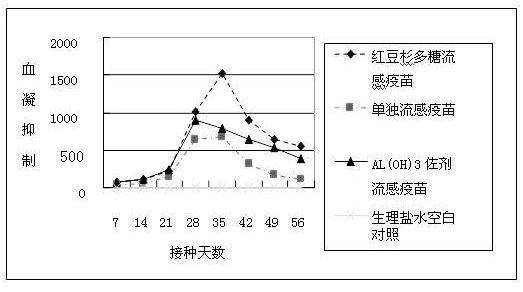
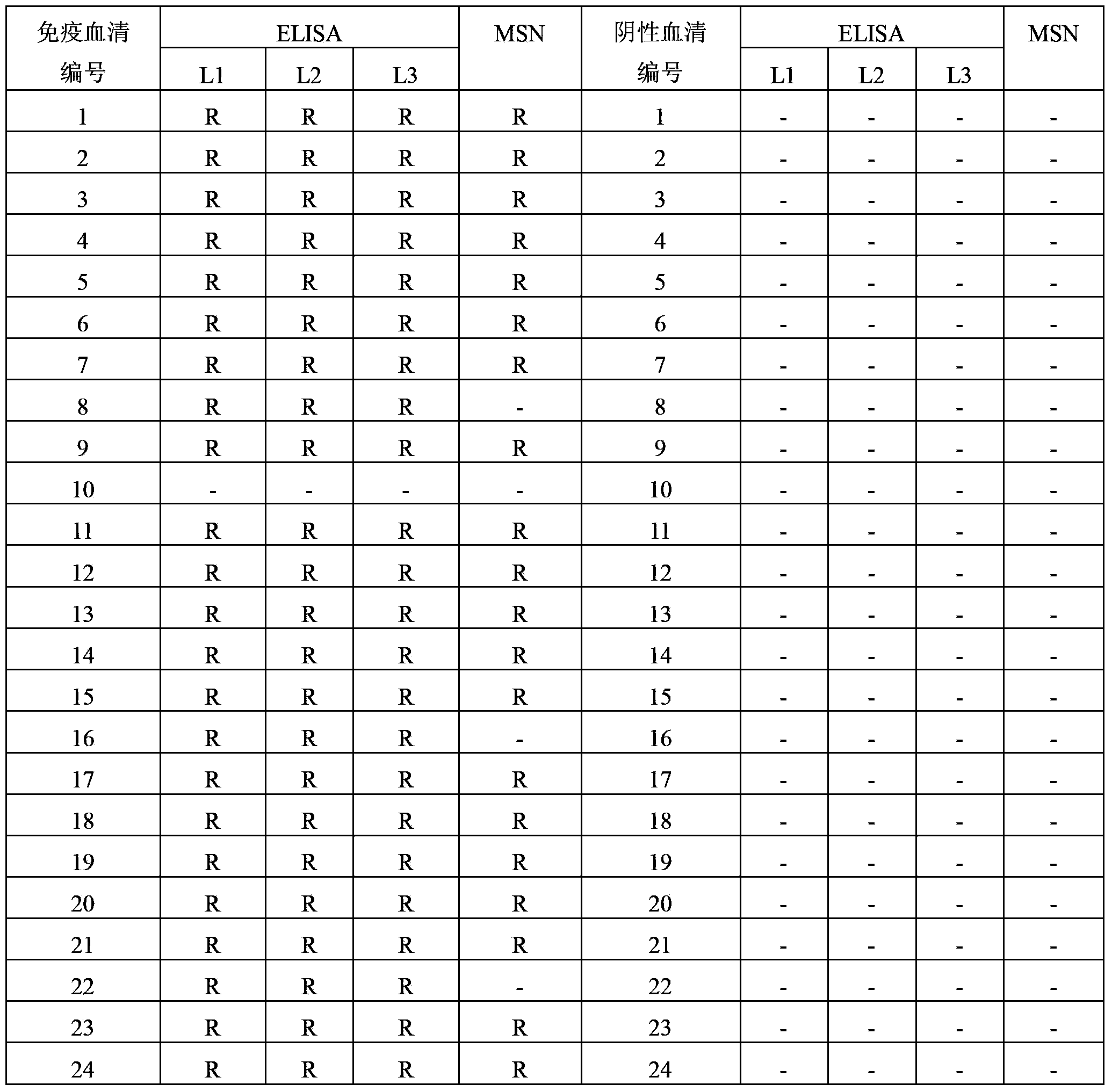
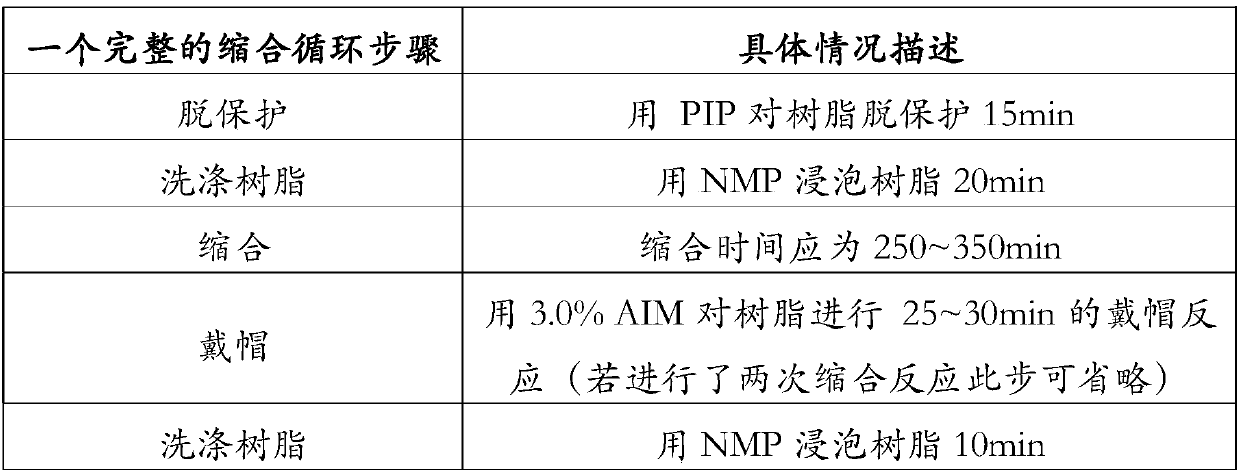
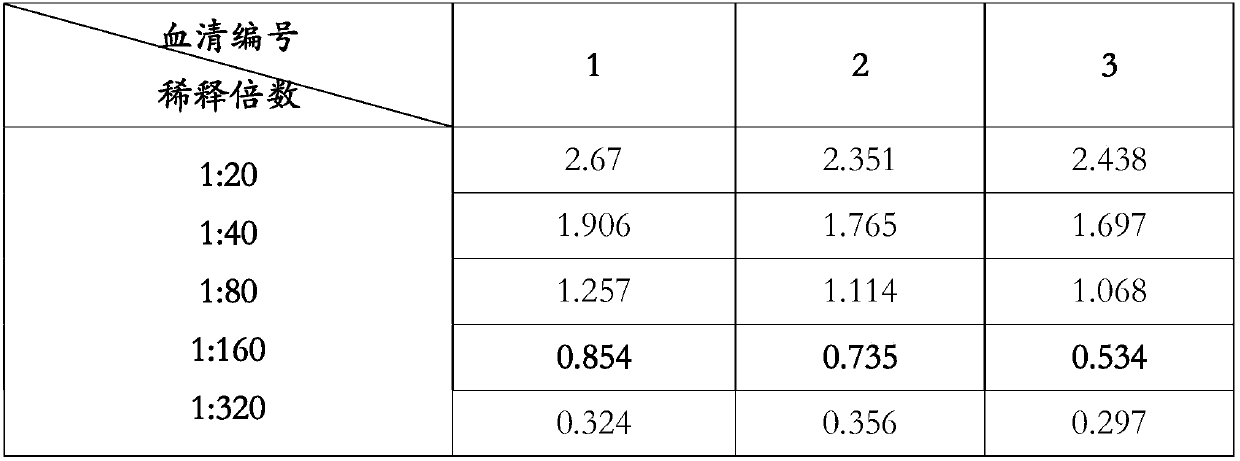
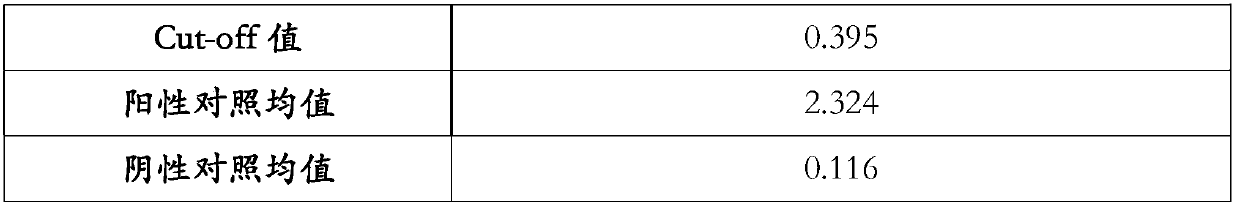
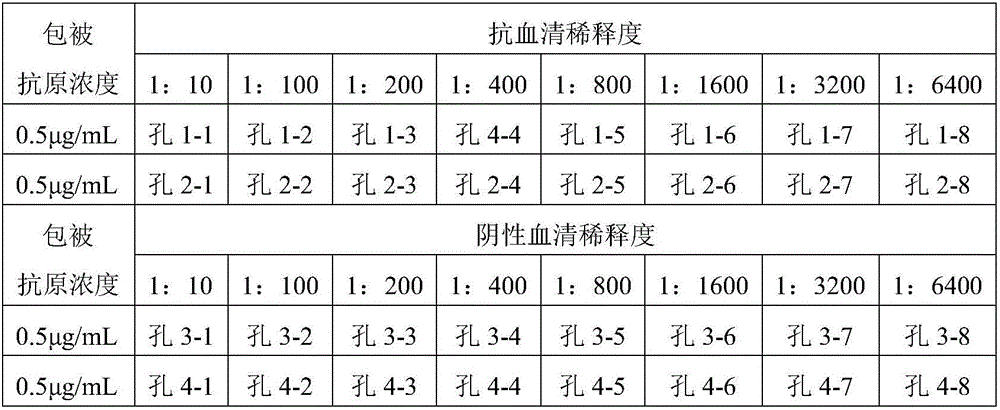
Patents
Literature
56results about How to "Less antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
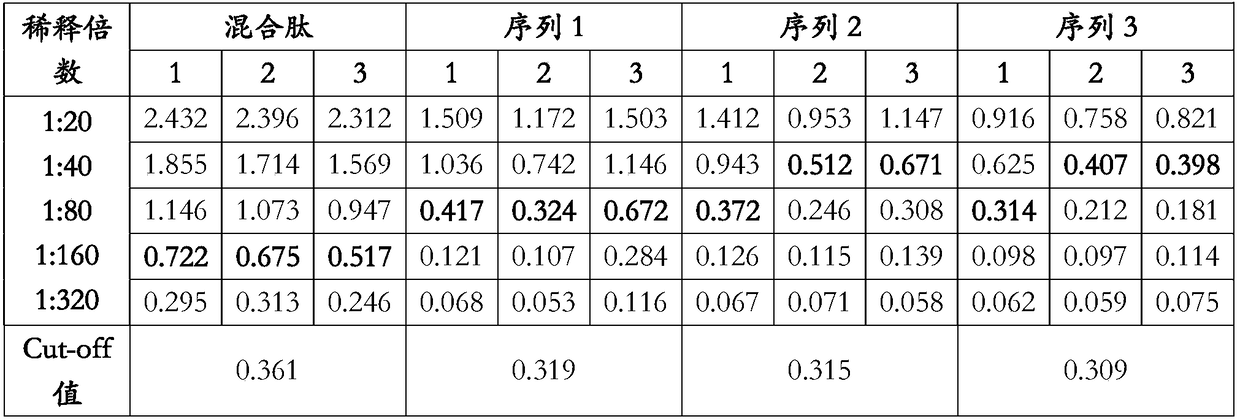
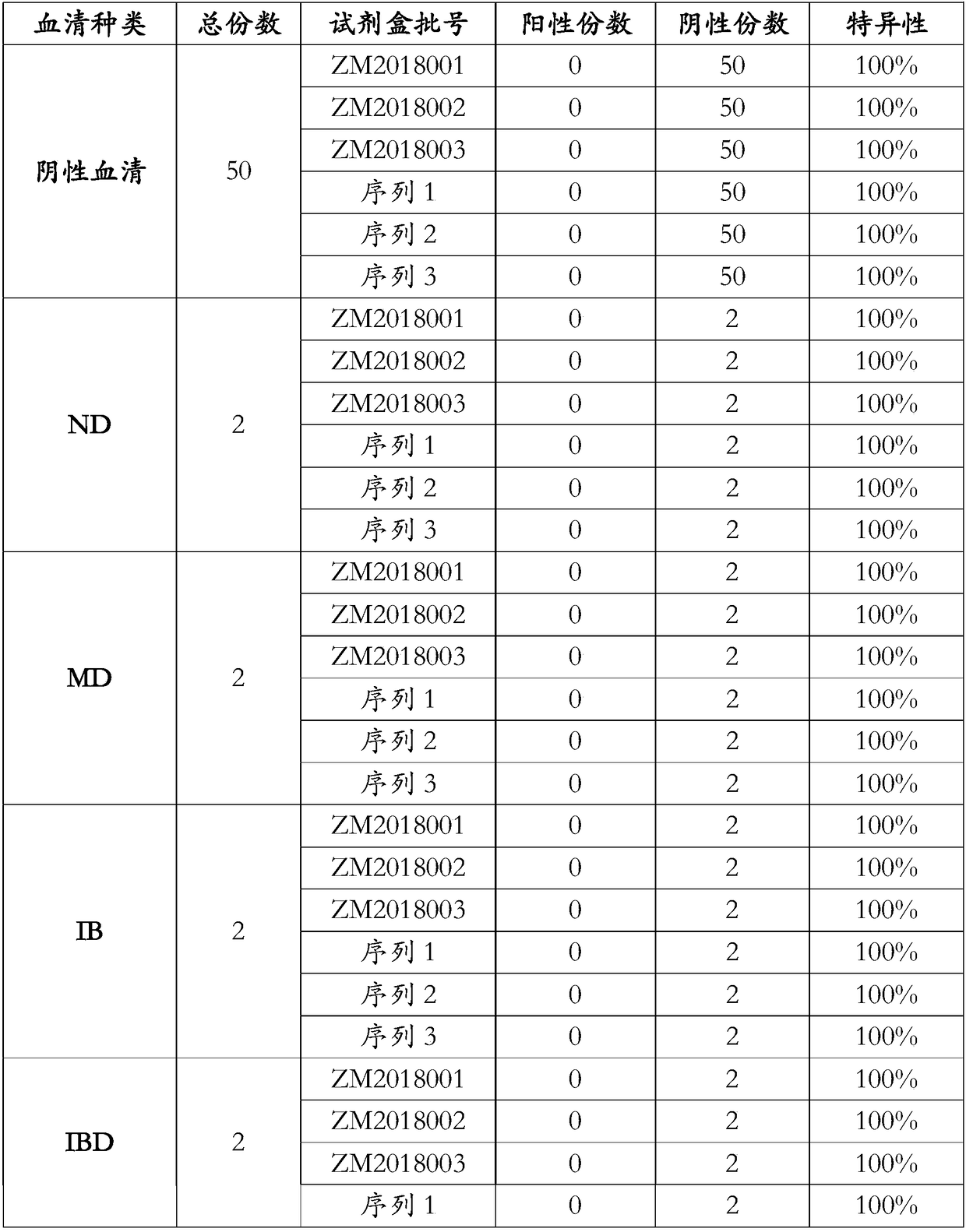
Enzyme-linked immunoassay kit of structural protein antibody for seneca valley virus
ActiveCN107253978AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisPositive control
The invention discloses an enzyme-linked immunoassay kit of a structural protein antibody for a seneca valley virus. The kit comprises an elisa plate, positive control serum, negative control serum, an HRP-conjugated antibody, a sample diluent, a 20-fold concentrated detergent, a substrate solution A, a substrate solution B and a stop solution, wherein the elisa plate is coated with a structural protein epitope polypeptide composition for the seneca valley virus. The epitope polypeptide composition is one or any combination of more than two of a polypeptide as shown in a sequence 1, a polypeptide as shown in a sequence 2, a polypeptide as shown in a sequence 3 or a polypeptide as shown in a sequence 4 in the sequence table. The elisa plate is coated with a chemical synthetic antigen peptide, so that the kit is low in antigen dosage and high in sensitivity and specificity, and whether the structural protein antibody is infected by the seneca valley virus or not can be efficiently detected. The kit is high in sensitivity, good in specificity, convenient in operation, and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
African swine fever virus synthetic peptide ELISA antibody detection kit
ActiveCN110642925AIncreased sensitivityStrong specificityVirus peptidesBiological material analysisClassical swine fever virus CSFVEpitope
The invention discloses an African swine fever virus synthetic peptide ELISA antibody detection kit. The kit includes an enzyme-labeled plate, a positive control serum, a negative control serum, an enzyme-labeled secondary antibody, a sample dilution solution, a 20-fold concentrated washing solution, a substrate solution A, a substrate solution B and a stop solution. The enzyme-labeled plate is coated with African swine fever virus epitope polypeptide composition. The epitope polypeptide composition is any combination of one or two or more of a polypeptide shown as a sequence 1 in a sequence listing, a polypeptide shown in a sequence 2 in the sequence listing and a polypeptide shown as a sequence 3 in the sequence listing. The kit uses a chemically synthesized antigen peptide-coated enzyme-labeled plate with low antigen consumption, high sensitivity and specificity, and can efficiently detect whether African swine fever virus antibodies exist or not. The kit is high in sensitivity, good in specificity, convenient to operate and good in market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Immunological adjuvant containing fucosan sulfate and application of immunological adjuvant
InactiveCN104383534AImproving immunogenicityGood effectAntiviralsAntibody medical ingredientsSide effectSulfate
The invention relates to an immunological adjuvant containing fucosan sulfate and application of the immunological adjuvant. The technical problems that at present, the kinds of immunological adjuvants are few, side effects cannot be avoided, and a great number of vaccines need to be provided when infectious diseases break out are solved. The immunological adjuvant consists of the following components in mass ratio: fucosan sulfate, ginsenoside and the rest of carriers, wherein the carriers consist of the following components in mass ratio: an emulsifying agent, an emulsifying auxiliary agent, oil phase and aqueous phase. The immunological adjuvant can be widely used in the preparation field of vaccines.
Owner:WEIHAI RENSHENG PHARMA GRP
Taxus polysaccharide immunologic adjuvant and influenza vaccine containing same
ActiveCN102160893AImprove securityLittle side effectsAntiviralsAntibody medical ingredientsTaxusImmunogenicity
The invention provides a taxus polysaccharide immunologic adjuvant and an influenza vaccine containing the same. The taxus polysaccharide immunologic adjuvant consists of the following components in percentage by mass: 0.001 to 100 percent of taxus polysaccharide and 0 to 99.999 percent of carrier. A mass ratio of the taxus polysaccharide immunologic adjuvant to hemagglutinin of the influenza vaccine is 50 to 183,500. The taxus polysaccharide is a natural substance extracted from branches of taxus; when the taxus polysaccharide is used as the immunologic adjuvant, applicable experiment results are obtained, and the taxus polysaccharide has high safety and small side effect. The influenza vaccine containing the taxus polysaccharide immunologic adjuvant is easy and convenient to prepare, the quality of the vaccine is easy to control, the immunogenicity of the vaccine can be better improved, and the dosage of antigen of the vaccine is greatly reduced; experiments prove that the dosage ofthe antigen of the vaccine, which takes the taxus polysaccharide as the adjuvant, is only half or lower than half of the dosage of the antigen of the vaccine, which takes aluminum hydroxide as the adjuvant, but the immune protective effect is the same.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Antibody-secreting cell assay
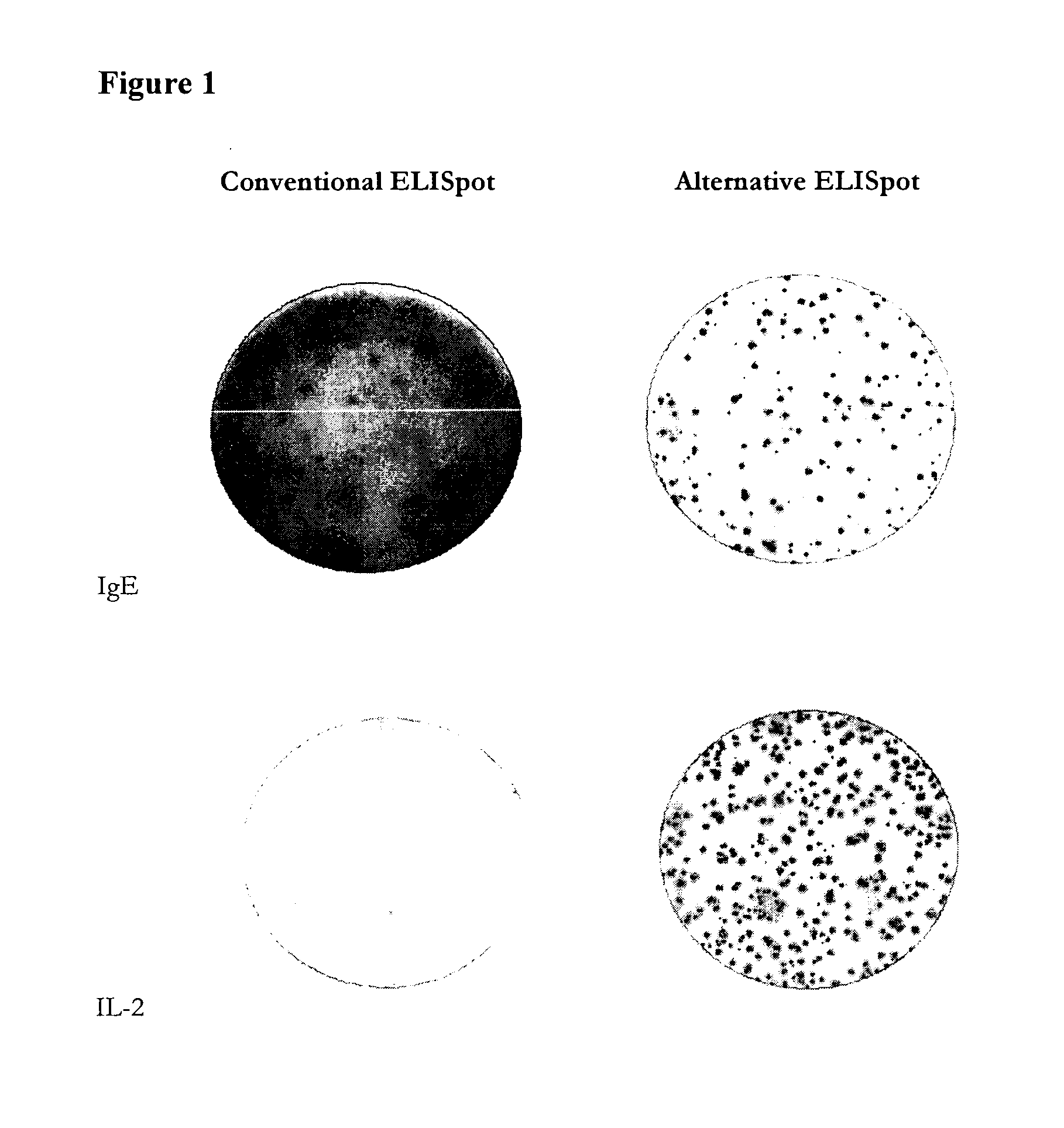
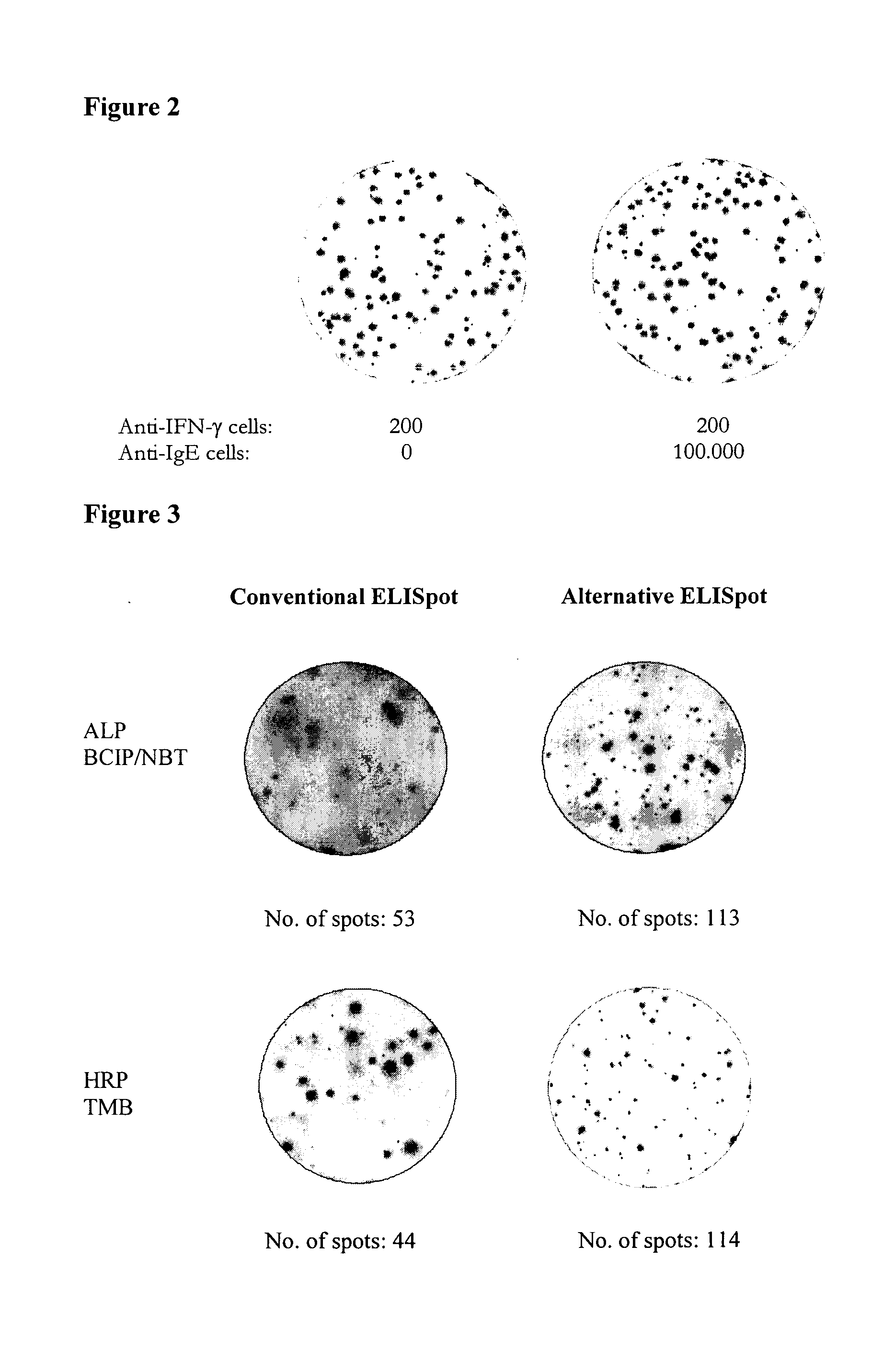
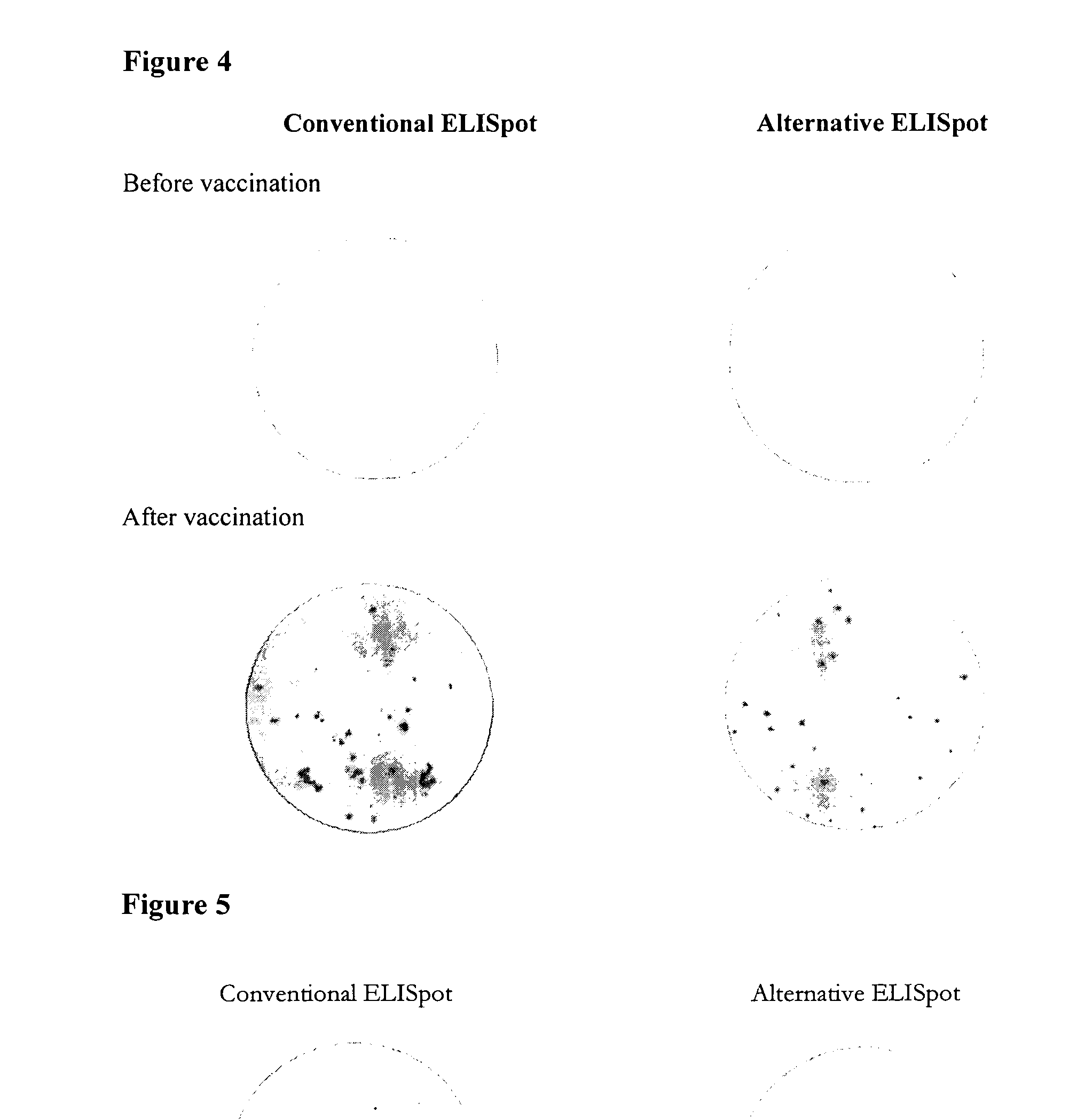
ActiveUS20110244477A1Less antigenLimited valueDisease diagnosisBiological testingEpitopeAntigen binding
An improved assay is described where a surface is provided with immobilized anti-Ig antibodies rather than antigen and where specific antibody-secreting cells (ASC) are detected using soluble antigen probes containing one of several possible labels. The method gives improved sensitivity with less background and is also more representative because antigen binding does not employ immobilized antigen. The assay is particularly effective for measuring antibody secreting cells against HIV, for determining whether an infection is acute as opposed to old or latent, for mapping epitopes and for measuring for ASCs against different antigens in the same reaction.
Owner:MABTECH
Foot and mouth disease virus structural protein antibody enzyme-linked immunosorbent assay kit
ActiveCN103408641AHigh purityStrong specificityVirus peptidesBiological material analysisFoot-and-mouth disease virusStructural protein
The invention discloses a foot and mouth disease virus structural protein antibody enzyme-linked immunosorbent assay kit. The kit comprises an enzyme-linked reaction plate coated by foot and mouth disease virus structural protein VP1 antigenic epitope polypeptides, and an enzyme-labeled antibody. The foot and mouth disease virus structural protein VP1 antigenic epitope polypeptides are a polypeptide represented by the sequence 1 in the sequence list and a polypeptide represented by the sequence 2 in the sequence list. According to the kit, the reaction plate coated by chemically synthesized VP1 antigenic peptide, antigen dose is low, sensitivity is high, and specificity is high. With the kit, whether foot and mouth disease virus infection exists can be highly efficiently detected. The kit provided by the invention has good specificity, high sensitivity, high efficiency, and good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Seneca valley virus structural protein epitope polypeptide and application thereof
ActiveCN109627293AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
The invention discloses a Seneca valley virus structural protein epitope polypeptide and application thereof. The polypeptide is the polypeptide represented by a sequence 1 in a sequence table, a sequence 2 in the sequence table, a sequence 3 in the sequence table or a sequence 4 in the sequence table. The Seneca valley virus structural protein epitope polypeptide is used for preparing a chemically synthesized antigen peptide coated elisa plate used for a kit, is low in antigen dosage and high in sensitivity and specificity, can effectively detect whether a structural protein antibody infectedwith a Seneca valley virus exists or not, is high in sensitivity, good in specificity and fast and convenient to operate and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Adjuvanted formulations of rabies virus immunogens
InactiveUS20150030630A1Higher immune titersImprove protectionSsRNA viruses negative-senseViral antigen ingredientsAdjuvantTlr agonists
The efficacy of rabies vaccines can be enhanced by adjuvanting rabies virus immunogens with a mixture of a TLR agonist (preferably a TLR7 agonist) and an insoluble metal salt (preferably an aluminium salt). The TLR agonist is typically adsorbed to the metal salt. The rabies virus immunogen can also be adsorbed to the metal salt.
Owner:NOVARTIS AG
Method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant
InactiveCN101745109AImmunity-boosting propertiesLess antigenAerosol deliveryAntiviralsInfluenza virus vaccineLysis
The invention provides a method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant, which belongs to the technical field of biology. The method comprises the following steps: adopting chicken embryo to culture proliferative influenza virus; obtaining purified influenza virus through ultrafiltration concentration and column chromatography; obtaining influenza vaccine stock solution through lysis; and proportioning the influenza vaccine stock solution of a required type to CpG ODN adjuvant or I:G adjuvant. The invention provides the method for preparing novel nasal-spray type influenza virus vaccine, which can reduce the amount of influenza virus antigen, provides convenience for immunization and vaccination, and is suitable for rapidly improving the capacity of supplying influenza vaccine under threat of global pandemic influenza.
Owner:云南沃森生物技术股份有限公司
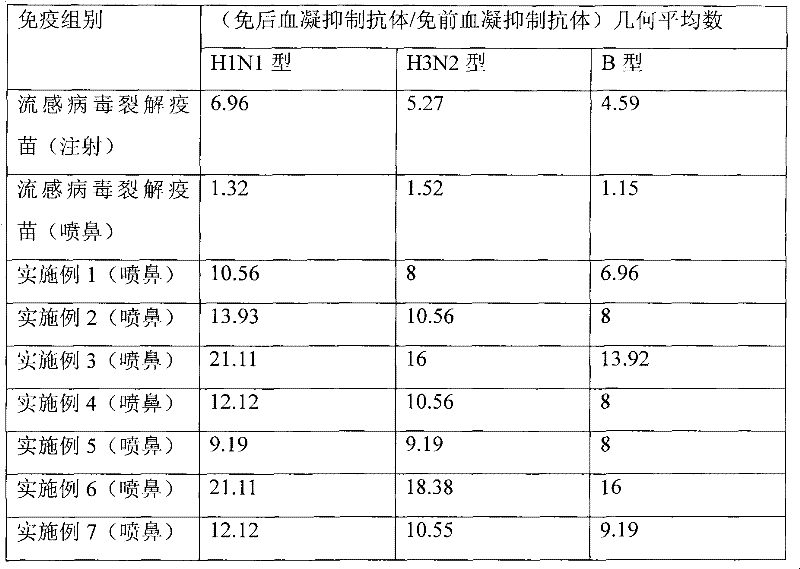
Nasal-spraying immune influenza multivalent vaccine and preparation method thereof
InactiveCN101450208AEasy to useLess antigenPowder deliveryAerosol deliveryHighly Pathogenic Avian Influenza VirusInfluenza a
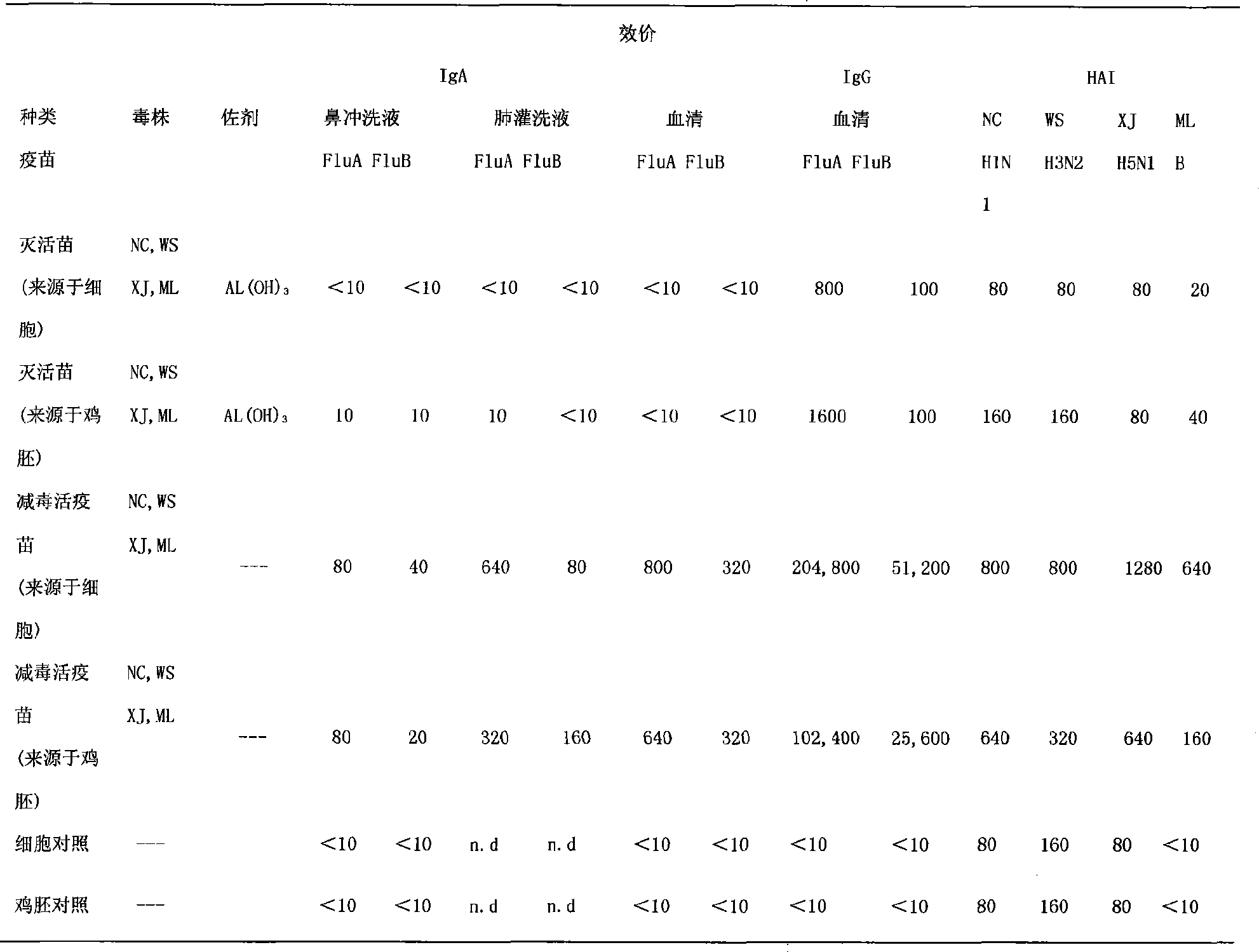
The invention discloses a nasal spray immunity flu polyvalent vaccine and a preparing method. Flu quadrivalence (H1N1, H3N2, H5N1, B type) or polyvalent attenuated live vaccine is used for immunoprophylaxis through nose spraying, popular flu and highly pathogenic avian influenza can be prevented safely and efficiently.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
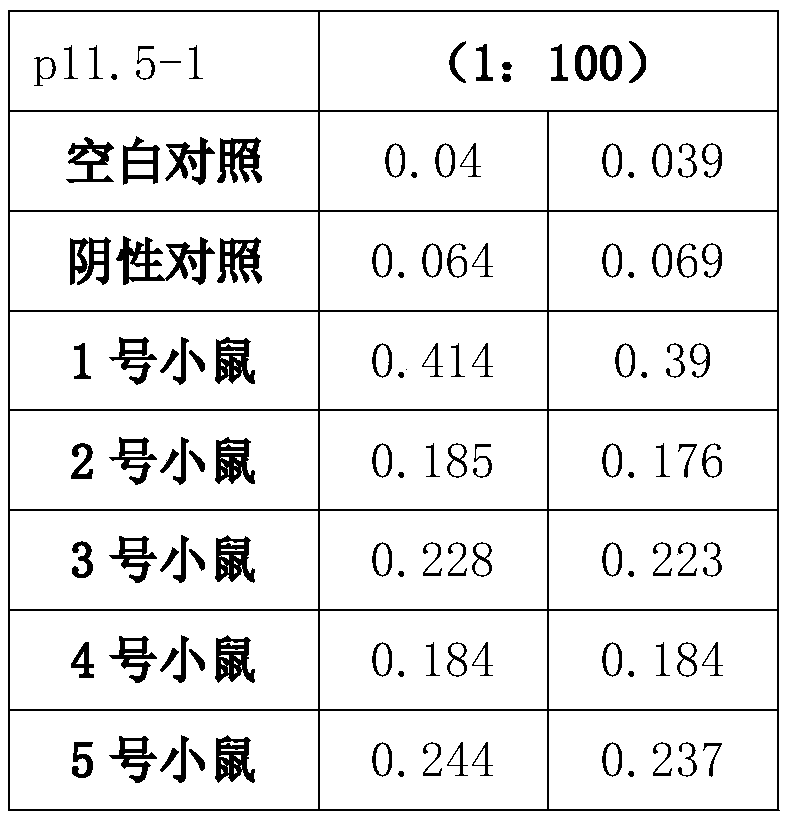
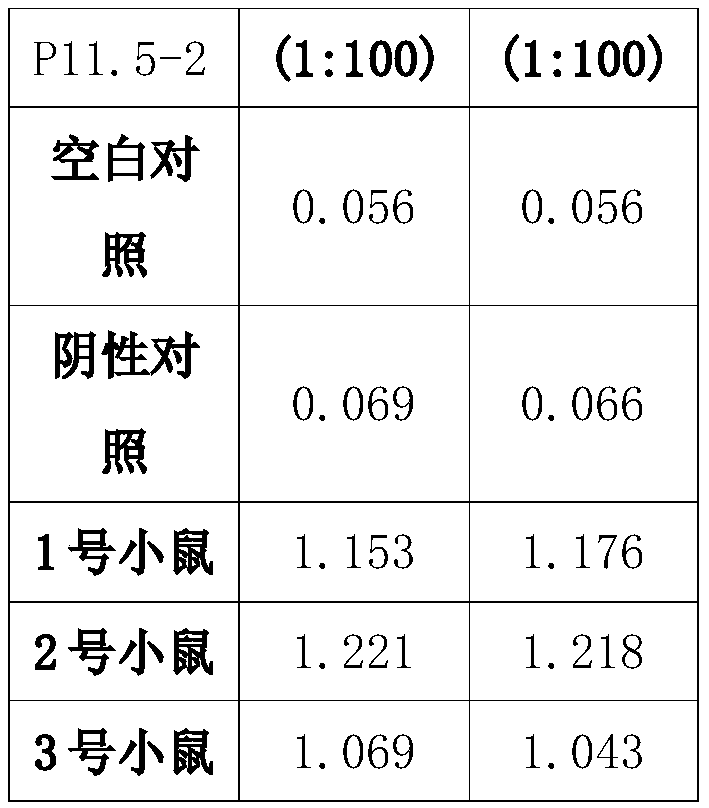
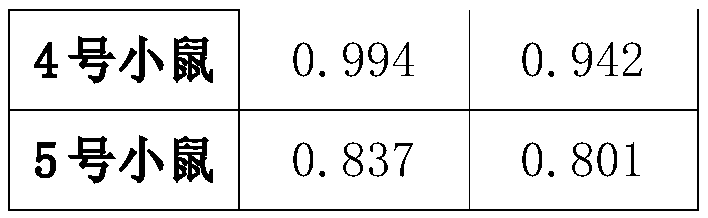
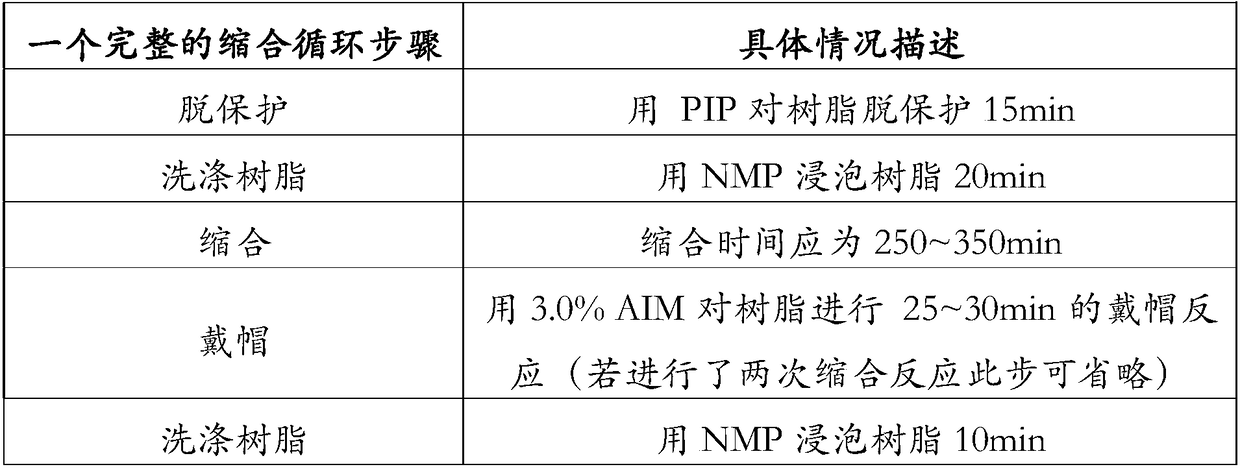
Preparation method and application of African swine fever virus P11.5 protein specific polyclonal antibody
ActiveCN109836478AStrong specificityHigh affinitySerum immunoglobulinsVirus peptidesEpitopeAfrican swine fever
The present invention discloses a preparation method and an application of an African swine fever virus P11.5 protein specific polyclonal antibody. The method uses P11.5 polypeptide epitope immunological experimental animals of mice, rabbits, etc. to prepare the specific polyclonal antibody. The method has high antigen purity, the prepared antibody is strong in specificity and high in affinity, used antigen is small in amount, and operations are simple and convenient. The preparation method of the polyclonal antibody comprises steps of ASFV P11.5 specific antigen epitope selection, polypeptideantigen synthesis, animal immunity, antibody determination, etc. Compared with conventional methods, the preparation method is simple and rapid, and high in antigen purity, the prepared antibody is strong in specificity, and the used antigen is small in amount. The polyclonal antibody can be used for detection of African swine fever virus P11.5 protein and provides an important technical method for prevention and control of African swine fever in China.
Owner:YANGZHOU UNIV
Avian influenza virus H9 subtype antibody enzyme-linked immunoassay kit
The invention discloses an avian influenza virus H9 subtype antibody enzyme-linked immunoassay kit. The kit comprises an enzyme-labelled plate, a positive control serum, a negative control serum, an enzyme-labelled second antibody, a sample diluent, a sample diluent, a 20-fold concentrated washing solution, a substrate solution A, a substrate solution B and a stop solution, wherein the enzyme-labelled plate coats an avian influenza virus H9 subtype HA protein epitope polypeptide composition. The epitope polypeptide composition is any combination of one or more than two of polypeptide represented by a sequence 1 in a sequence list, polypeptide represented by a sequence 2 in the sequence list or polypeptide represented by a sequence 3 in the sequence list. The avian influenza virus H9 subtype antibody enzyme-linked immunoassay kit in the invention adopts an indirect method ELISA and uses a chemical synthetic antigen peptide to coat the enzyme-labelled plate; the use amount of antigen islow, and the sensitivity and the specificity are high; it can be effectively detected whether the avian influenza virus H9 subtype antibody exits. The kit in the invention has advantages of high sensitivity, good specificity, convenience for operations and good market aspect.
Owner:CHINA ANIMAL HUSBANDRY IND
Method for producing goat-anti-human cystatin C protein antiserum
InactiveCN105061599AStable productionLow costSerum immunoglobulinsImmunoglobulins against protease inhibitorsSerum igeTiter
The invention provides a method for producing a goat-anti-human cystatin C protein antiserum; the method includes the following steps: (1) antigen preparation; (2) first injection; (3) second injection; (4) third injection; (5) fourth injection; (6) fifth injection; (7) sixth injection; (8) seventh injection; (9) eighth injection; (10) serum taking; and (11) serum titer detection: taking the antiserum, and detecting the titer. Compared with the prior art, the method for producing the goat-anti-human cystatin C protein antiserum is low in cost and simple in process, and not only can stably produce antibodies, but also can has fast antibody producing speed; and the antibody level is high and the antigen dosage is small.
Owner:海奥斯生物科技镇江有限公司
Nonstructural protein antibody ELISA (enzyme-linked immunosorbent assay) Kit for porcine foot-and-mouth disease virus
ActiveCN107513101AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
The invention discloses a nonstructural protein antibody ELISA (enzyme-linked immunosorbent assay) kit for a porcine foot-and-mouth disease virus. The kit comprises a foot-and-mouth disease virus nonstructural protein epitope polypeptide coated enzyme-linked reaction plate and an enzyme labeled antibody; foot-and-mouth disease virus nonstructural protein epitope polypeptides are a polypeptide shown as sequence 1, a polypeptide shown as sequence 2, a polypeptide shown as sequence 3 and a polypeptide shown as sequence 4 in a sequence table. According to the kit, a chemically synthesized nonstructural protein antigen peptide is used for coating the reaction plate, antigen dosage is small, sensitivity and specificity are high, and the presence of foot-and-mouth disease virus infection can be effectively detected. The kit provided by the invention has good specificity, sensitivity and high efficiency, and has good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Inactivation method for snake venom and inactivated snake venom prepared thereby
InactiveCN102241760ALow toxicityLess antigenPeptide preparation methodsAnimals/human peptidesAntigen epitopeNeutralising antibody
The invention relates to an inactivation method for snake venom. According to the method, snake venom is alkylated by a protein alkylating reagent, and therefore snake venom is inactivated. Preferably, alkylation is carried out under reversible denaturation conditions and finished after lucifugal culturing for 1 to 10 hours at a temperature of 20 to 40 DEG C; the protein alkylating reagent is iodoacetamide, iodacetic acid or iodoacetate; the alkylation is carried out in a buffer solution for snake venom; the concentration of snake venom is 0.01 to 100 mg / ml; and snake venom is venom of viper or pallas pit viper, cobra, long-noded pit viper and coral snakes. The invention also relates to inactivated snake venom prepared by the inactivation method. The method is novel and enables no obvious change of conformation of snake venom and retention of critical antigen epitope; therefore, when inactivated snake venom is immunized, high titer neutralizing antibodies can be generated, the amount of antigen for immunization is reduced, side reactions are avoided, rapid generation of high titer neutralizing antibodies is obtained, and the method is suitable for large scale popularization and application.
Owner:SHANGHAI SERUM BIOTECH
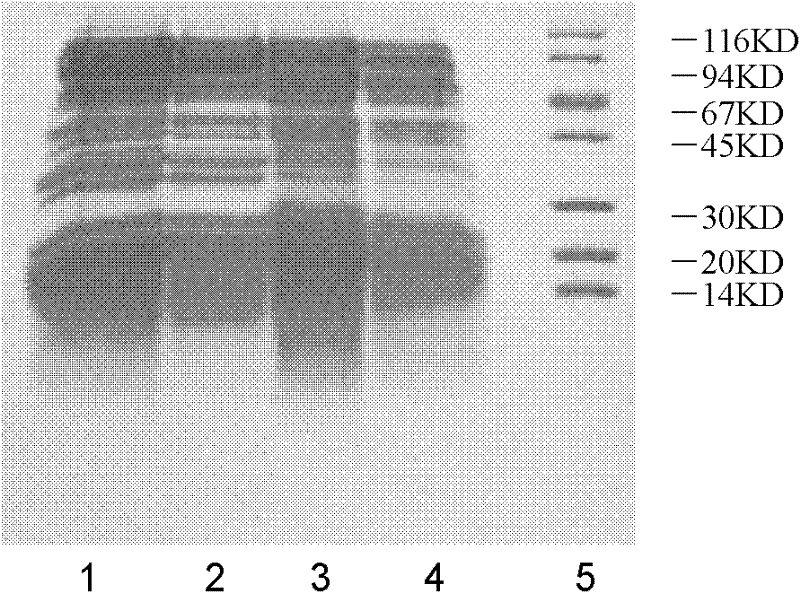
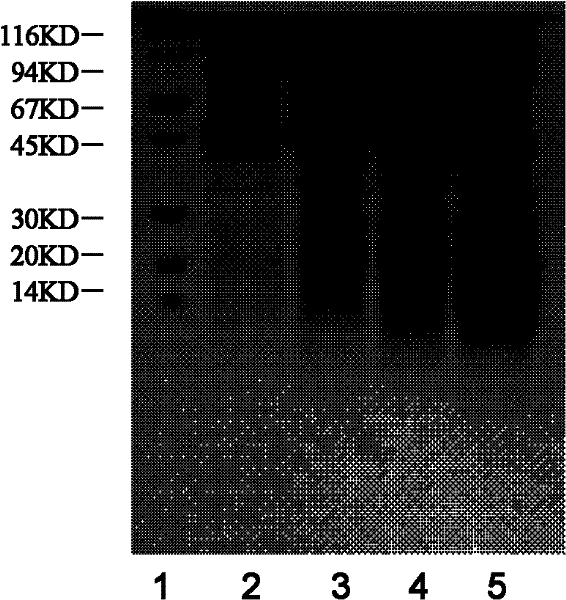
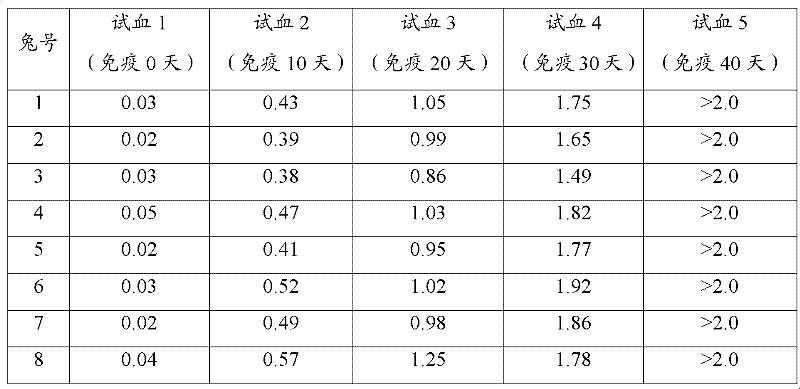
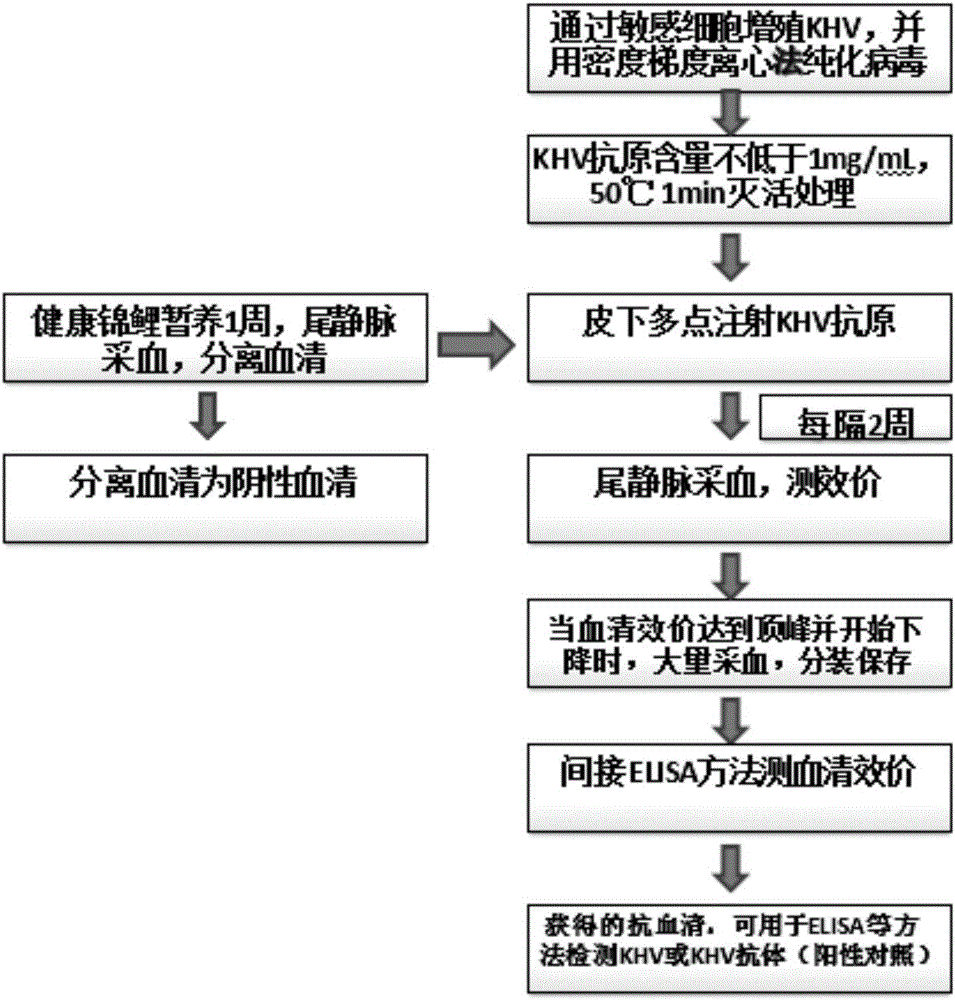
Preparation method and application of Koi herpes virus antiserum
InactiveCN106279407ASimplify vaccination proceduresLess antigenSerum immunoglobulinsImmunoglobulins against virusesAntiserumTiter
The invention relates to a preparation method of antiserum, and in particular to a preparation method and application of Koi herpes virus antiserum. The preparation method comprises the following steps: selecting healthy Koi as an experimental animal, collecting purified Koi herpes virus antigens, subcutaneously injecting at multiple points of the back of the Koi, when a serum titer reaches a peak value, sampling a great amount of blood from heart or caudal veins, separating serum, measuring a titer, split charging, freezing, and storing. The obtained Koi herpes virus antiserum is used for detecting a KHV (Koi herpes virus) antigen and can be used as positive serum of the KHV antibody detection method. According to the preparation method of the Koi herpes virus antiserum, a preparation method of Koi KHV resistant antiserum is established, the high-titer antiserum is obtained, and the obtained antiserum can be used for detecting the KHV antigen in methods such as IFAT, ELISA and the like and can be used as the positive serum to detect the KHV antibody. A programmed antiserum preparation method is provided for performing the KHV or KHV antibody immunology diagnosis in China, and a finished product immunology diagnosis reagent can be provided.
Owner:LIANYUN PORT IMMIGRATION INSPECTION & QUARANTINE BUREAU PEOPLES REPUBLIC OF CHINA
Spirulina polysaccharide immune adjuvant and influenza vaccine containing the same
ActiveCN102028945AEasy to prepareReduced activityAntiviralsAntibody medical ingredientsSide effectVaccine antigen
The invention provides a spirulina polysaccharide immune adjuvant and an influenza vaccine containing the same, wherein the spirulina polysaccharide immune adjuvant is prepared from the components in mass ratio as follows: 0.001-100% of spirulina polysaccharide and 0-99.999% of carrier, which is capable of preferably improving immunogenicity of the vaccine and greatly reducing dosage of antigen vaccine; the dosage of the antigen vaccine taking the spirulina polysaccharide as adjuvant is only a half of the dosage of the antigen vaccine taking the aluminum hydroxide as adjuvant or less, which can achieve ideal immune protection effect as well with high safety and small side effect; the influenza vaccine taking the spirulina polysaccharide as adjuvant is convenient for preparation and the quality thereof is easy to control.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Immunogenic composition
ActiveCN102333541ALess antigenReduce the number of dosesPharmaceutical non-active ingredientsImmunological disordersAdjuvantMicroparticle
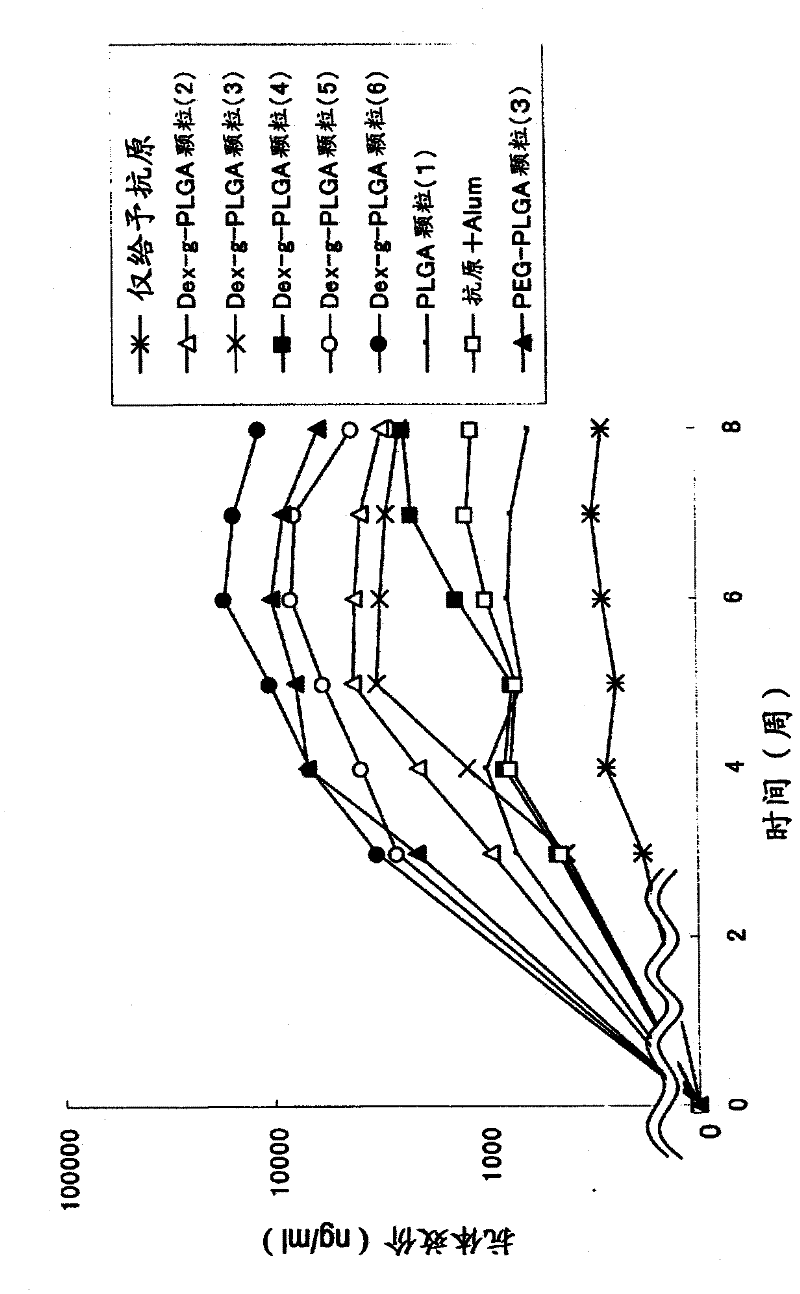
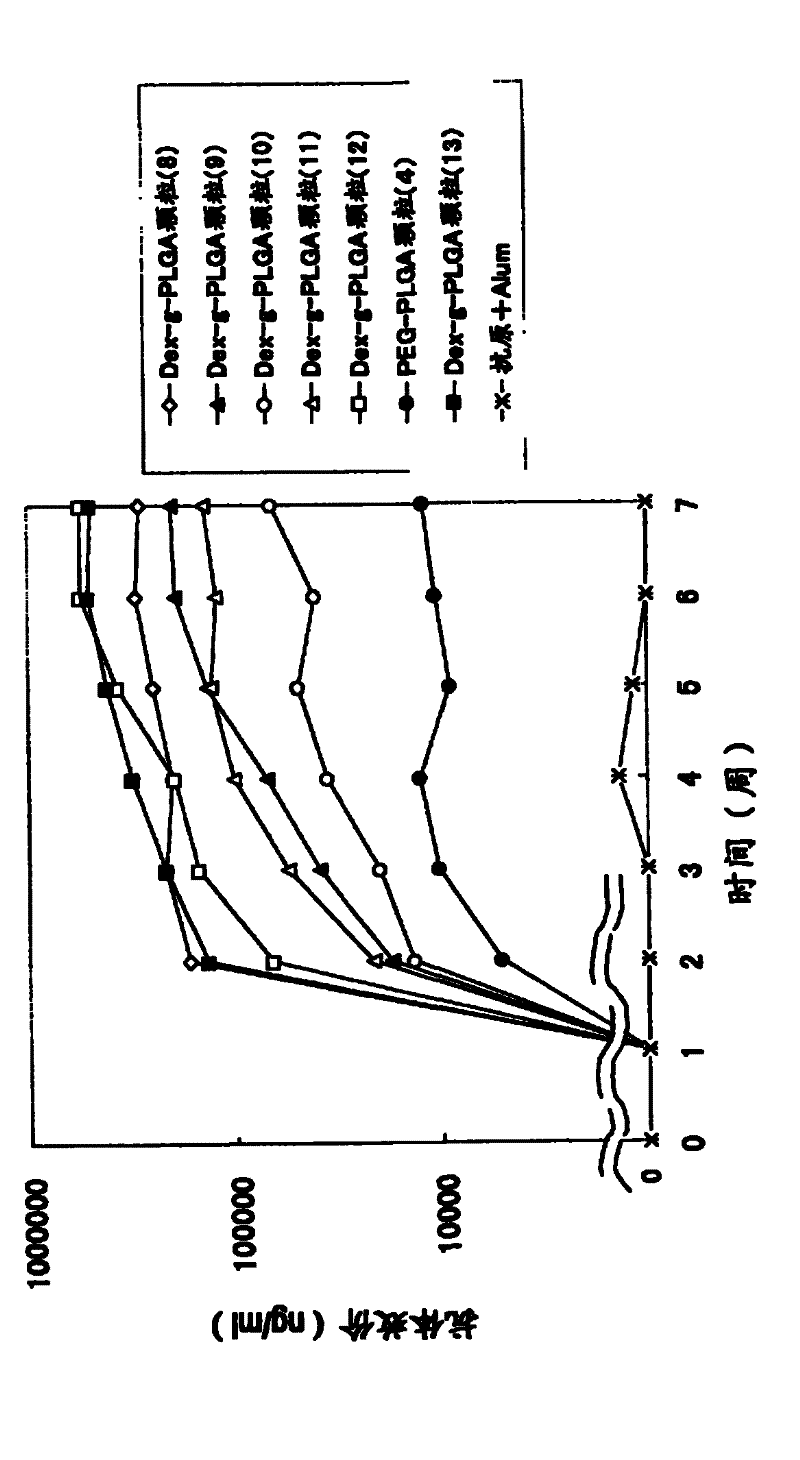
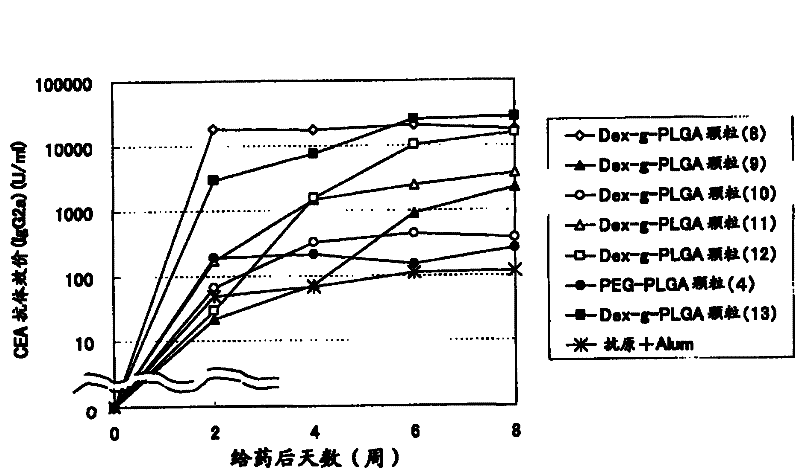
Disclosed is an antigen-adjuvant microparticle complex which comprises adjuvant microparticles and an antigen encapsulated in the adjuvant microparticles, wherein each of the adjuvant microparticles comprises an amphipathic polymer which has a poly(hydroxy acid) as a hydrophobic segment. Also disclosed is an immunogenic composition which contains particles conjugated with the complex as an active ingredient. The complex or the immunogenic composition can induce a high immune response to a small quantity of an antigen or can induce a high immune response to an antigen by a few frequencies of administration, and is therefore useful as a vaccine that is effective for the treatment and prevention of infectious diseases, cancer and others.
Owner:TORAY IND INC
Turtle shell immunoadjuvant and influenza vaccine containing the same
ActiveCN101926994AEasy to prepareReduced activityAntiviralsAntibody medical ingredientsHemagglutininDisease
The invention relates to a turtle shell immunoadjuvant and an influenza vaccine containing the immunoadjuvant; the turtle shell immunoadjuvant comprises the following ingredients in percentage by weight: 0.001-100% of turtle shell extractive and 0-99.999% of carriers. The composition of the influenza vaccine containing the turtle shell immunoadjuvant is as follows: the mass ratio of the turtle shell immunoadjuvant to the influenza vaccine hemagglutinin is 50-180050. The turtle shell immunoadjuvant in the invention has a good immunoadjuvant effect and has an effect of effectively preventing diseases in human and animal vaccine. The turtle shell extractive is used as the immunoadjuvant of the influenza vaccine; compared with the traditional aluminum hydroxide immunoadjuvant, the turtle shell immunoadjuvant has high using safety and small byeffect, can well improve the original nature of the influenza vaccine; in the process of preparing the influenza vaccine, the method is simple, economic and environment friendly and convenient; the method easily controls the mass, greatly reduces the dosage of the influenza vaccine antigen and satisfies the immunity demand when occurring the epidemic influenza.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI +1
Application of chitosan in avian vaccine composition preparation
InactiveCN102973936AImprove the immunityLess antigenViral antigen ingredientsAntiviralsOil phaseVaccine antigen
The invention relates to an application of chitosan in avian vaccine composition preparation. An avian vaccine composition comprises newcastle disease inactivation virus or avian influenza inactivation virus and other avian vaccine antigens, and a chitosan solution, wherein the avian vaccine comprises a water phase and an oil phase, the chitosan solution is in the water phase, and a concentration of the chitosan solution is 0.1-0.5% W / V, most preferably 0.2-0.5% W / V. According to the avian vaccine composition, the amount of the antigen is low so as to reduce side reactions of the avian vaccine and reduce avian vaccine cost, such that the avian vaccine composition such as newcastle disease vaccines or avian influenza vaccines and the like are suitable for industrial production.
Owner:PU LIKE BIO ENG
ELISA antibody detection kit for African swine fever viruses MGFs and CD2v
ActiveCN111961120AIncreased sensitivityStrong specificityVirus peptidesBiological testingClassical swine fever virus CSFVEpitope
The invention discloses an ELISA antibody detection kit for African swine fever viruses MGFs and CD2v. The kit comprises an ELISA plate and an enzyme-labelled secondary antibody, wherein the ELISA plate is coated with an African swine fever virus epitope polypeptide composition. The epitope polypeptide composition is one or arbitrary combination of two or more of polypeptide represented in the sequence 1 in the sequence table, polypeptide represented in the sequence 2 in the sequence table and polypeptide represented in the sequence 3 in the sequence table. The kit adopts chemically synthesized antigen peptide to coat the ELISA plate, is low in antigen dosage and high in sensitivity and specificity, and can efficiently detect whether MGF360-505R and CD2v antibodies produced by wild strainsinfected with the African swine fever viruses exist or not, and thereby realizing differential diagnosis. The kit is high in sensitivity, good in specificity and convenient to operate and has good market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Preparation of antigen sensitized human dendron shaped cell and its use
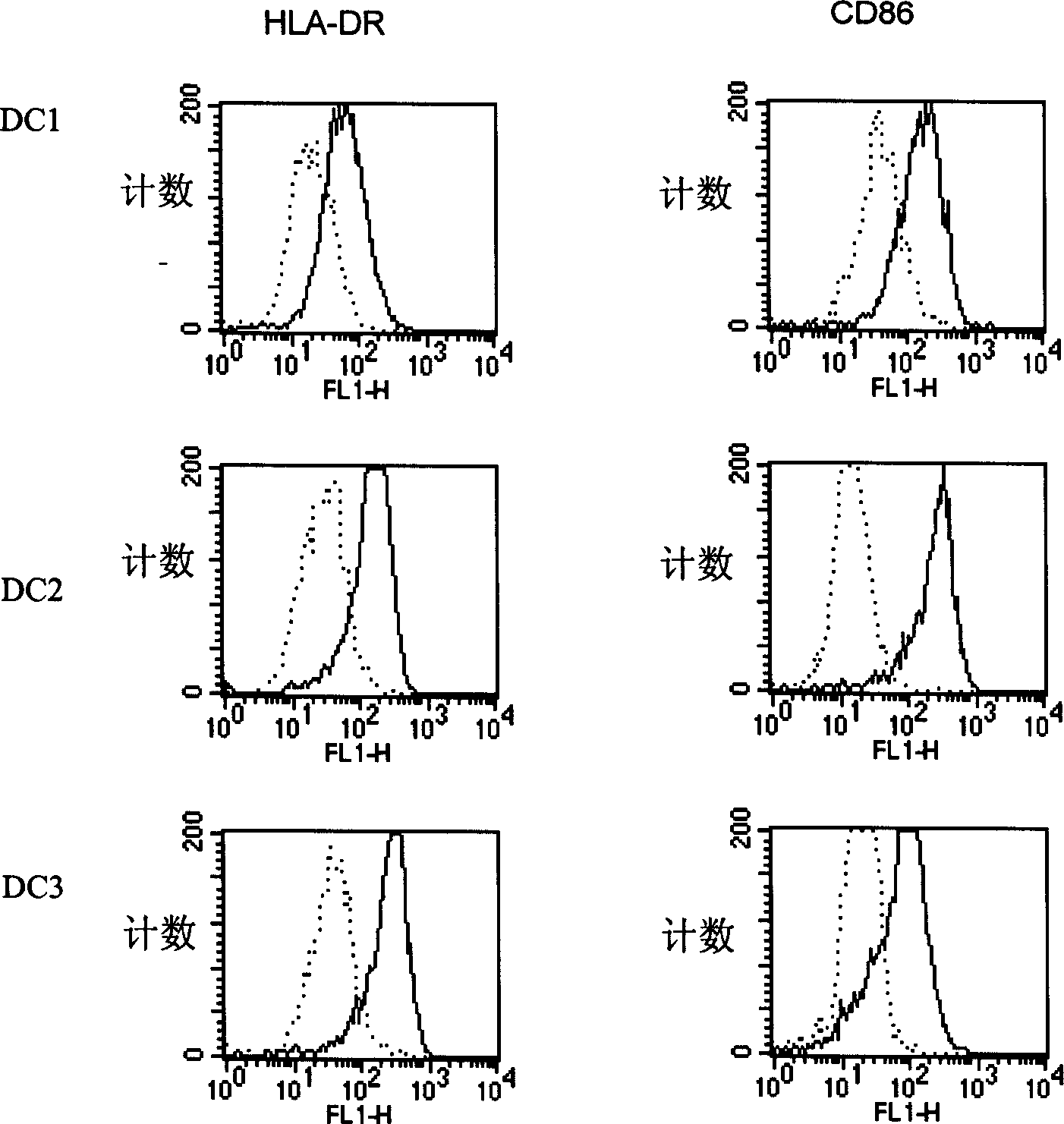
InactiveCN1475498AIdeal wayAvoid pollutionTissue cultureAntibody medical ingredientsDendritic cellMicrobiology
A method for sensibilizing the human dendritic cell, a process for preparing the antigen-sensitive human dendritic cell and storing it, and its application in antineoplastic immune are disclosed. Itsadvantages are high sensibilizing efficiency, long storage time, and high reviral rate after freeze storage.
Owner:上海海欣生物技术有限公司
Preparation method of mouse anti-human SAA monoclonal antibody
The invention belongs to the technical field of monoclonal antibodies and particularly relates to a preparation method of a mouse anti-human SAA monoclonal antibody. The method comprises the followingsteps of (1) antigen preparation; (2) animal immunization; (3) cell fusion, wherein preparation work including preparation of spleen cells and preparation of a feeding layer is carried out before fusion; (4) screening and cloning of hybridoma cells; (5) expanded production of the monoclonal antibody; (6) determination of the type, subtype and ascites titer of the monoclonal antibody. In step 2, amulti-point injection mode is adopted during first immunization and second immunization, and an emulsified antigen is used and injected into the intradermal cells, foot pads and the abdominal cavityseparately; at the same time, shock immunization is performed once three days before fusion. According to the preparation method, a multi-point combined immunization mode and a shock immunization modeare adopted, the antigen dosage is low, the process is simple, little damage is caused to mice, and the immunization effect is good. The preparation process of the feeding layer is optimized, the growth of the hybridoma cells is promoted, and the cell fusion rate and the positive rate are increased.
Owner:上海钹乐诗生物技术有限公司
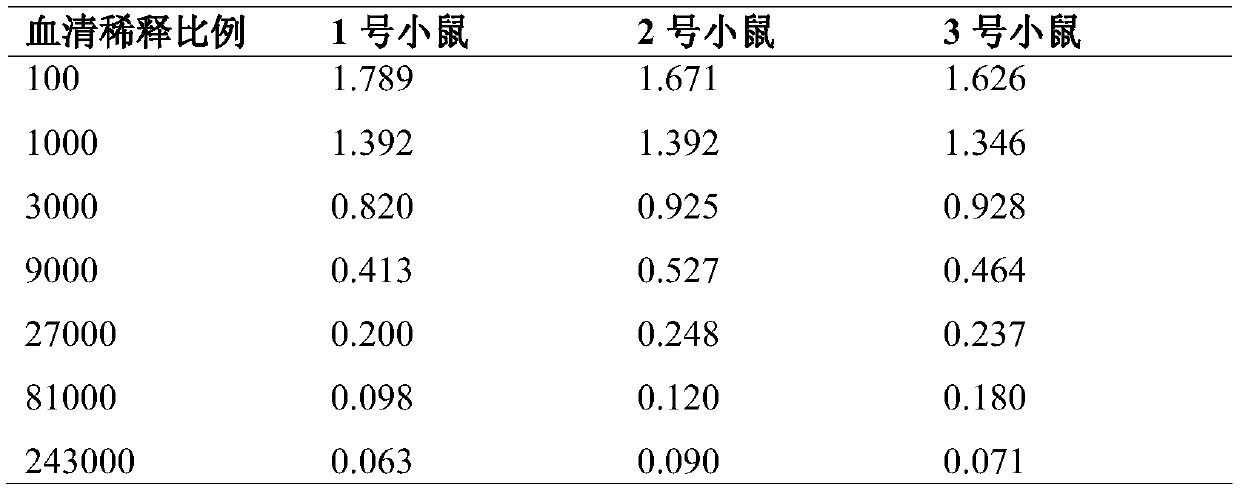
Method for preparing short and naked dinoflagellate toxin monoclone antibody
InactiveCN101509031ALow costImmunity is fastImmunoglobulins against fungi/algae/lichensFermentationAntigenSerum ige
A method for preparing a short-Gymnodinium toxin monoclonal antibody relates to the preparation of a monoclonal antibody in the immune safety detection technology, which is finished by the following steps: (1) the preparation of a complete antigen, comprising the preparation of an immune antigen (BTX-IgG) and a detection antigen (BTX-BSA); (2) animal immunization; (3) and the preparation of the monoclonal antibody by blending an immune mouse spleen cell with the serum titer greater than 10 and an SP2 / 0 myeloma cell according to a conventional method. The ascites titer of the monoclonal antibody which is prepared by the immune method can be up to 2.4*10, the affinity constant thereof is up to 0.6*10M, and the ascites titer and the affinity constant thereof are not lower than the ascites titer and the affinity constant of the antibody which is prepared by the traditional immune method; the immune speed is fast, thus the cost for preparing the monoclonal antibody is effectively reduced.
Owner:JILIN UNIV
Brucellosis vaccine needle-free injection system and application
InactiveCN105999253APrecision injectionFast injectionAntibacterial agentsBacterial antigen ingredientsAntigenImmune effects
The invention discloses a brucellosis vaccine needle-free injection system and application. The brucellosis vaccine needle-free injection system comprises a container filled with a brucellosis vaccine and a readable carrier. Content recorded in the readable carrier includes that needle-free injection dosage of the brucellosis vaccine is not larger than the standard dosage of the brucellosis vaccine. Experiments prove that when a needle-free injection ampoule and a needle-free syringe are combined and used for immunization, the antigen dosage only accounts for part of a traditional intramuscular injection antigen dosage, and the same or better immune effect can be achieved. It is further indicated that a needle-free immune mode is an ideal strategy capable of being selected by the brucellosis immunization vaccine.
Owner:INNER MONGOLIA HUAXI BIOTECH
Mucosa-targeted immune enhancer for animals and its application in veterinary vaccines
ActiveCN108014333BEasy to operateFlexible operationImmunological disordersAntibody medical ingredientsAntigenImmunity
Owner:JIANGSU ACAD OF AGRI SCI
Kratom antigen as well as preparation method and application thereof
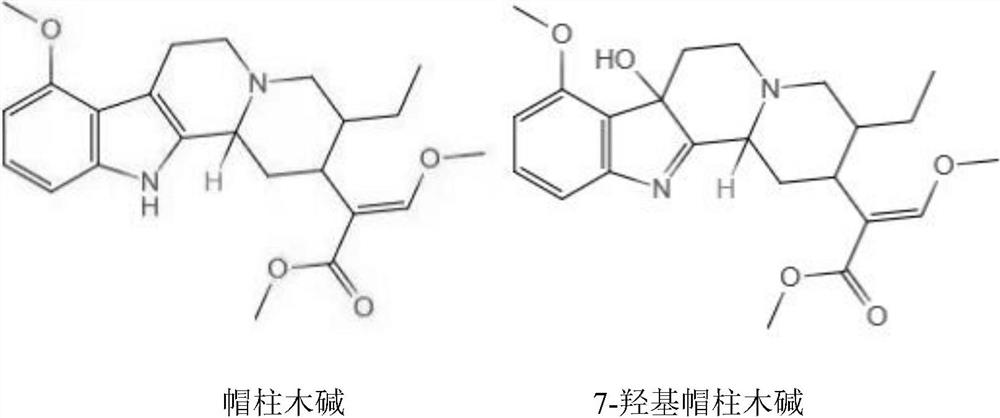
PendingCN113354725AMaintain structural specificityPromote productionSerum albuminImmunoglobulins against plantsMitragynineAntigen
The invention relates to the field of biological detection, and discloses a Kratom antigen as well as a preparation method and application of the Kratom antigen, the Mitragynine complete antigen and the 7-hydroxy Mitragynine complete antigen completely reserve active groups in molecular structures, so that the Kratom antigen has relatively strong specificity and relatively high sensitivity.
Owner:HANGZHOU CLONGENE BIOTECH
Foot-and-mouth disease virus non-structural protein antibody ELISA kit
ActiveCN103864906BStrong specificityHigh sensitivitySsRNA viruses positive-senseVirus peptidesChemical synthesisAntigen
The invention discloses a foot and mouth disease virus non-structural protein antibody enzyme-linked immunodetection kit. The kit comprises a foot and mouth disease virus non-structural protein 3B antigen epitope peptide-coated polymerase chain reaction plate and an enzyme-labeled antibody, wherein the foot and mouth disease virus non-structural protein 3B antigen epitope peptide is a polypeptide shown as a sequence 1 in a sequence table. The kit adopts a chemical synthesis non-structural protein 3B antigen peptide coated reaction plate and is small in antigen amount, high in sensitivity and high in specificity, and whether the foot and mouth disease virus infection exists can be efficiently detected. The kit is high in specificity, sensitive and high-efficiency and has good market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Porcine Foot-and-Mouth Disease Virus Nonstructural Protein 3abc Antibody ELISA Kit
ActiveCN104478998BIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
Owner:CHINA ANIMAL HUSBANDRY IND
Swine foot-and-mouth disease virus type A antibody enzyme-linked immunosorbent assay kit
ActiveCN110669112AHigh purityImproving the Efficiency of Detecting Foot-and-Mouth Disease AntibodiesSsRNA viruses positive-senseVirus peptidesAntigen epitopeAbzyme
The present invention discloses a swine foot-and-mouth disease virus type A antibody enzyme-linked immunosorbent assay kit. The swine foot-and-mouth disease virus type A antibody enzyme-linked immunosorbent assay kit comprises a foot-and-mouth disease virus type A antigen epitope polypeptide coated enzyme-linked reaction plate and an enzyme-labeled anti-antibody; and the foot-and-mouth disease virus type A antigen epitope polypeptide is a polypeptide shown in a sequence 1 or a polypeptide shown in a sequence 2 in a sequence listing. The kit uses the chemically synthesized antigen-peptide-coated reaction plate, is low in antigen consumption and high in sensitivity and specificity, and can efficiently detect presence of foot-and-mouth disease virus infection. The kit has good specificity, issensitive and high in efficiency, and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com