Nasal-spraying immune influenza multivalent vaccine and preparation method thereof
A technology of influenza vaccine and multivalent vaccine, applied in the field of vaccines, can solve the problems of immune protection without mucosal immune effect, achieve the effect of preventing highly pathogenic human avian influenza, avoiding pain and cross-infection, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of influenza quadrivalent (H1N1, H3N2, H5N1 and B type) attenuated live vaccine strain
[0037] Type A influenza virus strains H1, H3, H5 and type B influenza virus strains were provided by China CDC and Hualan Biotechnology Co., Ltd. Adopt the traditional chicken embryo allantoic cavity culture influenza virus method or microcarrier suspension culture and tame mammalian cells (such as Vero cells, MDCK cells, 2BS cells, etc.) to culture influenza virus.
[0038] ①Using the existing reverse genetics method, the cold-adapted attenuated influenza virus strain A / Ann Arbor / 6 / 60 was used as the virus backbone for type A polyvalent virus, and B / Ann Arbor / 1 / 66 was used as the backbone for type B polyvalent virus. Rescue the virus skeleton, integrate the HA and NA surface antigen genes of the popular influenza A and B strains of the year, and co-transfect lactation with 8, 3 or 4 recombinant plasmids constructed from H1N1, H3N2, H5N1 and B influenza...
Embodiment 2
[0042] Example 2: Preparation of influenza multivalent (H1N1, H3N2, B, H5N1, H7N7, H9N2 subtypes) live attenuated vaccine antigens
[0043] Type A influenza virus strains H1N1, H3N2, H5N1, H7N7, H9N2 subtypes and type B influenza virus strains were provided by China CDC and Hualan Biotechnology Co., Ltd. Adopt the traditional chicken embryo allantoic cavity culture influenza virus method or microcarrier suspension culture and tame mammalian cells (such as Vero cells, MDCK cells, 2BS cells, etc.) to culture influenza virus.
[0044] ①Using the existing reverse genetics method, the antigen of type A multivalent vaccine is based on the cold-adapted attenuated influenza virus strain A / Ann Arbor / 6 / 60 as the virus backbone, and the antigen of type B multivalent vaccine is based on B / Ann Arbor / 1 / 66 In order to save the virus skeleton, integrate the HA and NA surface antigen genes of influenza A and B strains that were popular in the year, and co-transfect mammals with 8, 3 or 4 reco...
Embodiment 3
[0048] Example 3: Observation of the in vivo effect of influenza quadrivalent live attenuated vaccine in mice
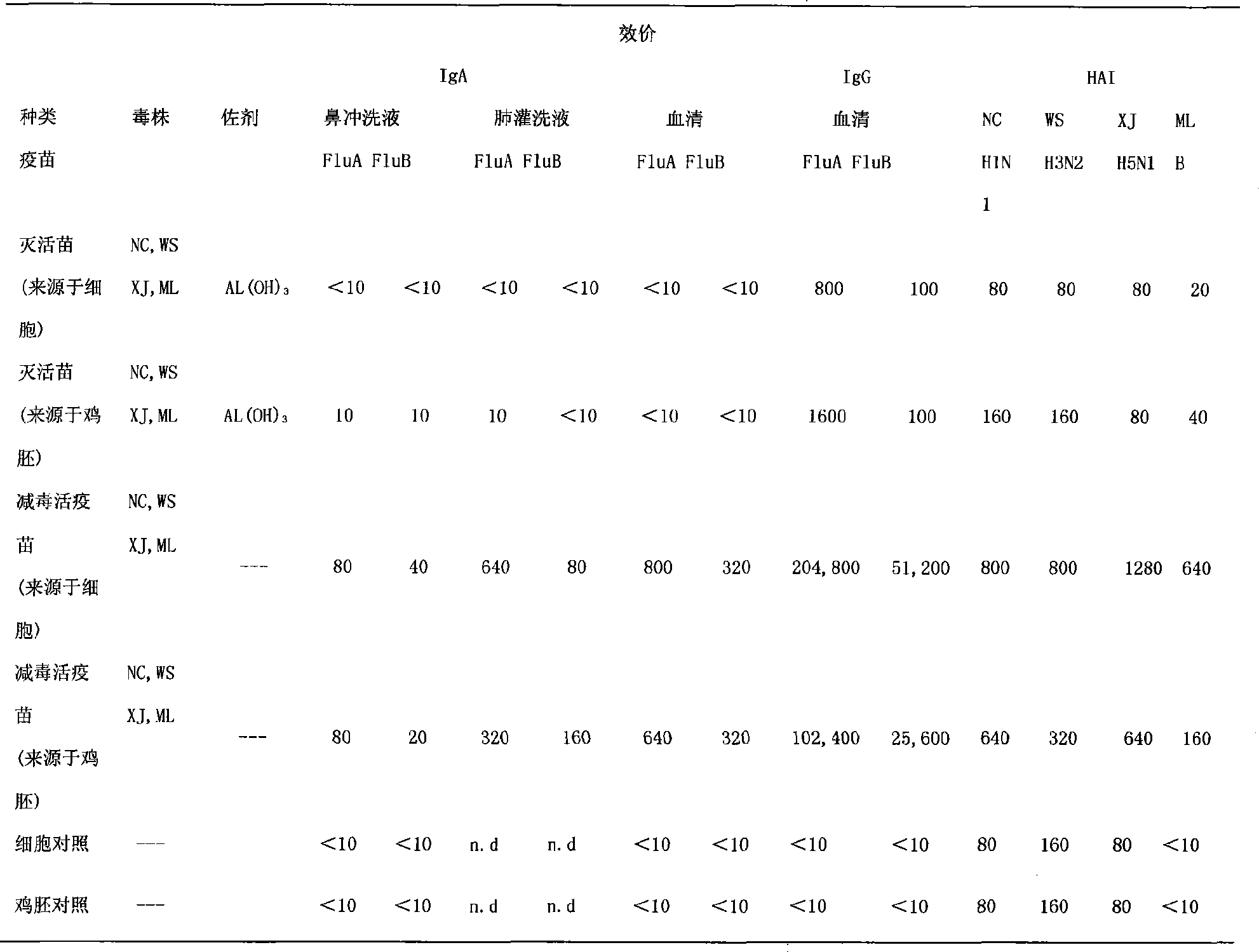
[0049] 1. Immunization strategy, using different dosage forms of influenza virus vaccine to immunize mice
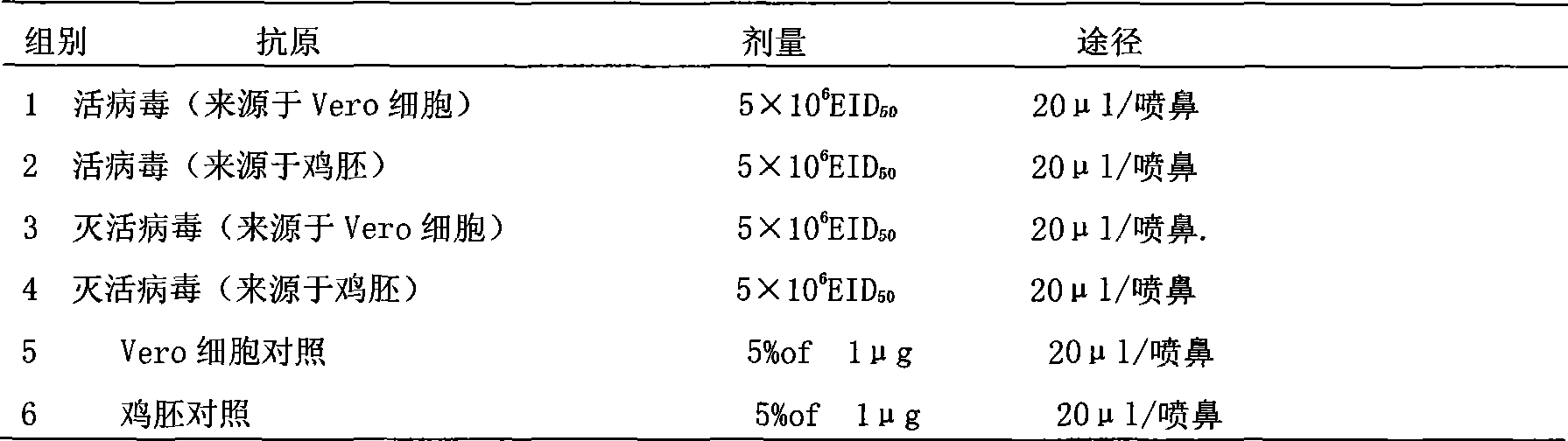
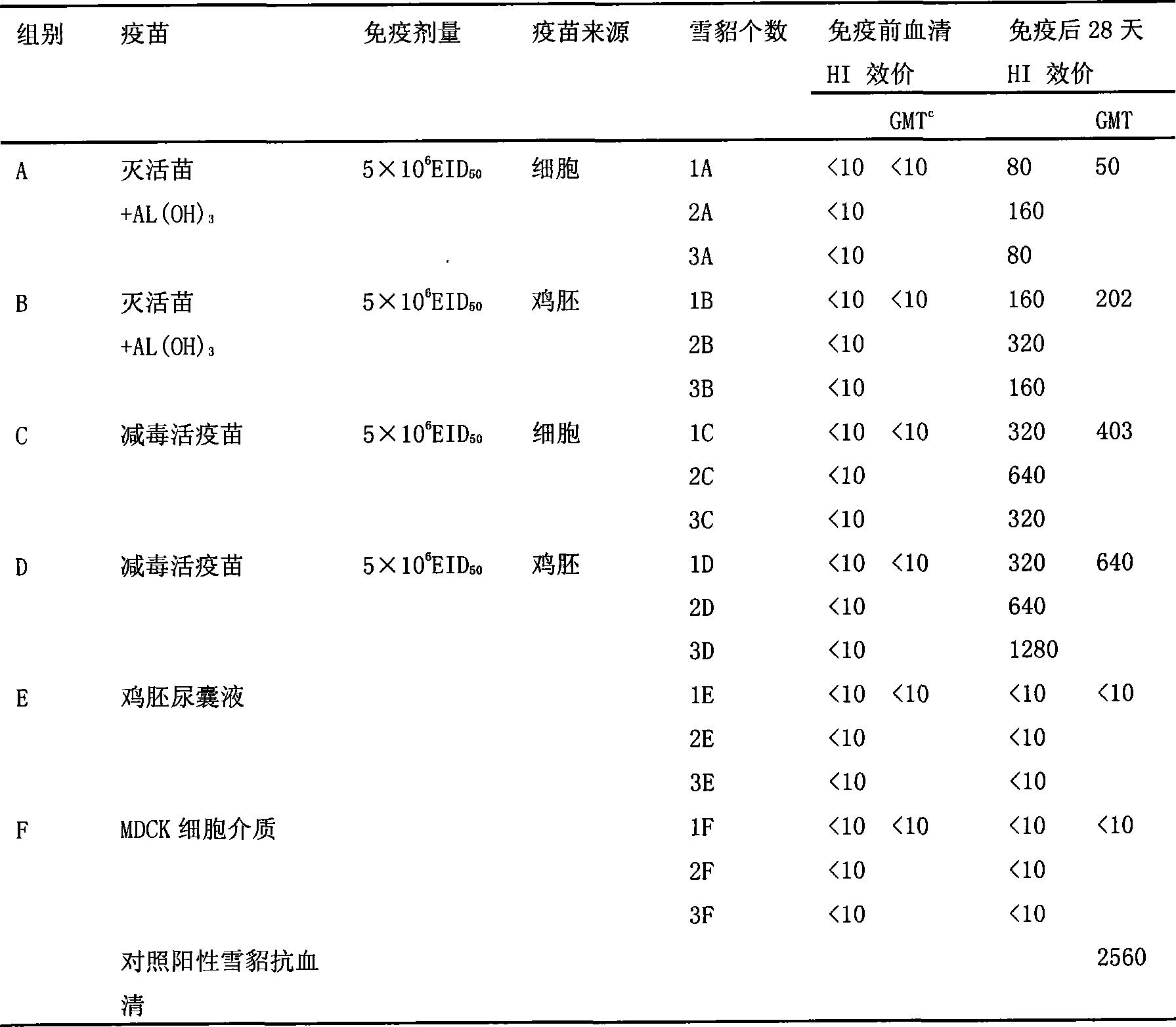
[0050] ① According to the method for preparing influenza quadrivalent (H1N1, H3N2, H5N1 and B type) attenuated live virus strains in Example 1, at the same time dilute the antigen with a stabilizer to the amount of the attenuated live vaccine ≥ 5 × 10 6 EID 50 , in which nasal inactivated influenza vaccine + AL(OH) 3 The adjuvant group was the control (Tab1).
[0051] ②The 6 groups of Balb / c mice received nasal immunization with vaccines of different dosage forms, 20 μl of antigen solution per mouse, in which the inactivated influenza vaccine contained AL(OH) 3 adjuvant. Immunization was carried out on the 0th and 28th day respectively, and the IgG, IgA and HAI titers in the serum, nasal washing fluid and lung washing fluid were measured on the 42nd day.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com