Immunogenic composition
A technology of immunogenicity and composition, which is applied in the field of immunogenic composition, can solve the problems of inconsistency in performance, slow-release performance, inference of adjuvant function, and failure to realize it, and achieve a powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] The synthesis of embodiment 1 dextran-poly(lactic acid-glycolic acid) (PLGA)
[0098] (1-1) Synthesis of TMS-dextran
[0099] Add dextran (Nacalai Tesque Co., Ltd., Nacalai standard super qualified product, number average molecular weight: 13,000, 5.0 g) into formamide (100 ml), and heat to 80°C. To this solution was added dropwise 1,1,1,3,3,3-hexamethyldisilazane (100 ml) over 20 minutes. After completion of the dropwise addition, the mixture was stirred at 80° C. for 2 hours. After the reaction, the reaction solution was returned to room temperature, and the two layers were separated with a separatory funnel. After concentrating the upper layer under reduced pressure, methanol (300 ml) was added, and the obtained solid was filtered and dried to obtain TMS-dextran (compound (1)) (11.4 g) as a white solid.
[0100] In the same manner, compounds (2) and (3) were prepared using dextran (manufactured by Sigma, average molecular weight 1,500 or less), and compounds (4) a...
Embodiment 2
[0108] The synthesis of embodiment 2PEG-PLGA (compound (10), (11))
[0109]Polyethylene glycol monomethyl ether (SUNBRIGHTMEH-20H manufactured by NOF Co., Ltd., number average molecular weight 5,128, Mw / Mn=1.02), (DL)-lactide and glycolide were mixed in the amount shown in Table 2, Heat to 140°C. After stirring for 20 minutes, tin (II) octoate (0.05% by weight based on polyethylene glycol monomethyl ether) was added, and stirred at 180° C. for 3 hours. After the reaction solution was returned to room temperature, it was dissolved in chloroform (to form a concentration of about 100 mg / ml), and purified by reprecipitation with diethyl ether cooled to 0°C, and the obtained solid was filtered off and dried under reduced pressure to obtain a white or pale The tan solid yielded PEG-PLGA polymer. The number average molecular weight of this polymer is given by 1 H-NMR determined (Table 2).
[0110] 【Table 2】
[0111] Table 2: Evaluation results of the prepared PEG-PLGA polymers ...
Embodiment 3
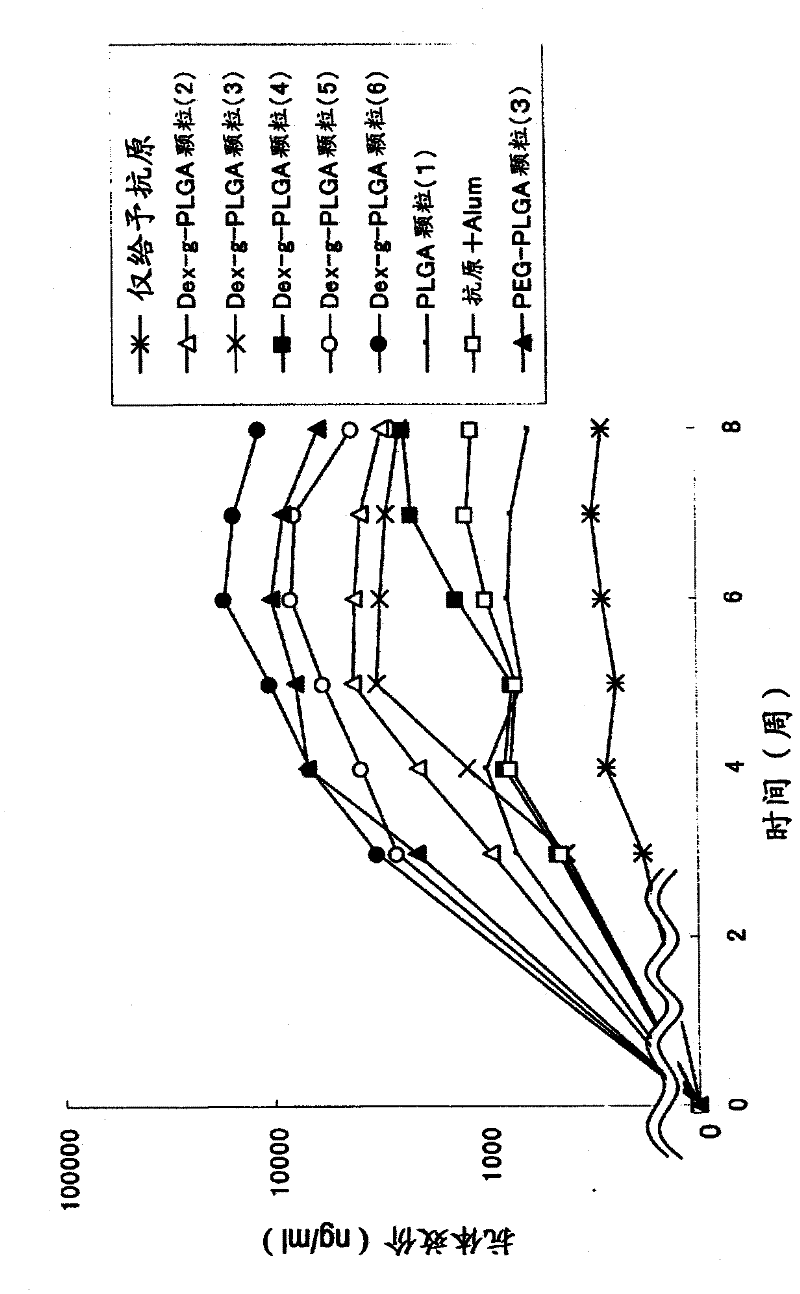
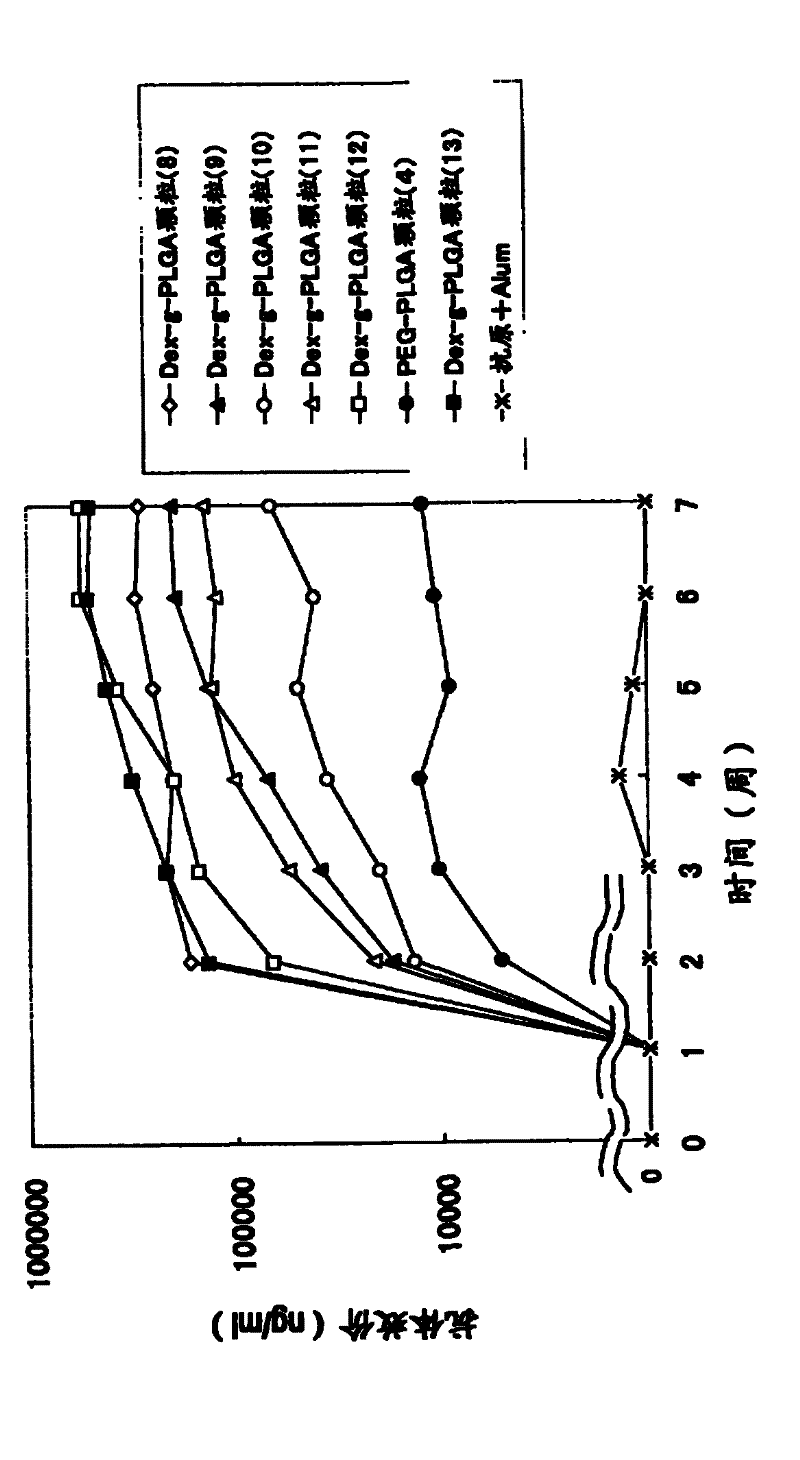
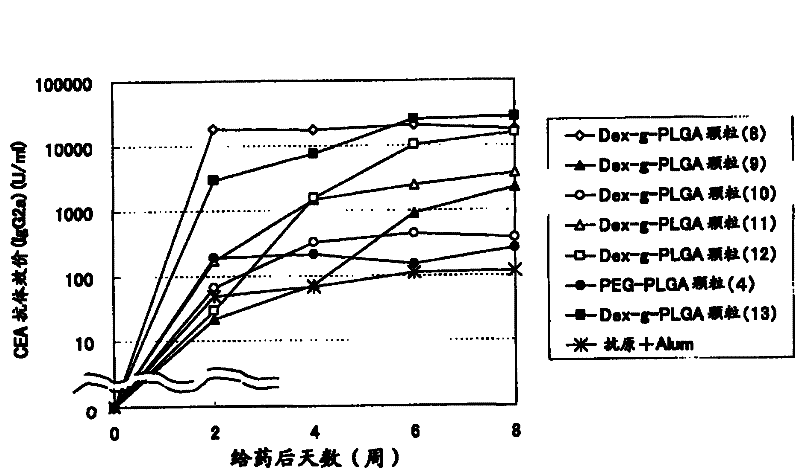
[0113] Example 3 Preparation of Antigen-Adjuvant Microparticle Complex Using Dex-g-PLGA Polymer (Dex-g-PLGA Particles (1)-(28))
[0114] 5 mg of dextran-poly(lactic-glycolic acid) (PLGA) (compounds (12) to (23)) of Example 1 was dissolved in 100 μl of dimethyl carbonate to prepare a 50 mg / ml polymer solution. After adding 20 μl of tert-butanol to the polymer solution, 50 μl of the embedded antigen (OVA (ovalbumin) (Sigma) or CEA (carcinoembryonic antigen) (COSMO) was added dropwise at the concentration recorded in Table 3. BIO company)) and immunoactive substances (CpG) were stirred with a VORTEX mixer to prepare an inverse emulsion. As CpG, 5'ggggggggCGACGATCGTCAgG-3' (small letters in the sequence indicate phosphorothioate modified bases) (commissioned by Sigma- Synthesized by Genosys Corporation).
[0115] The inverse emulsion was pre-frozen with liquid nitrogen, and then freeze-dried for 24 hours with a freeze dryer (EYELA, FREEZEDRYER FD-1000) at a trap cooling temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com