Application of fullerene derivative to prepare gene transmission vector
A technology of fullerene derivatives and gene transfer, which is applied in the field of biotechnology and vaccines, can solve the problems of poor water solubility of fullerenes, achieve the effect of improving defense capabilities, improving cellular immunity and humoral immunity, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
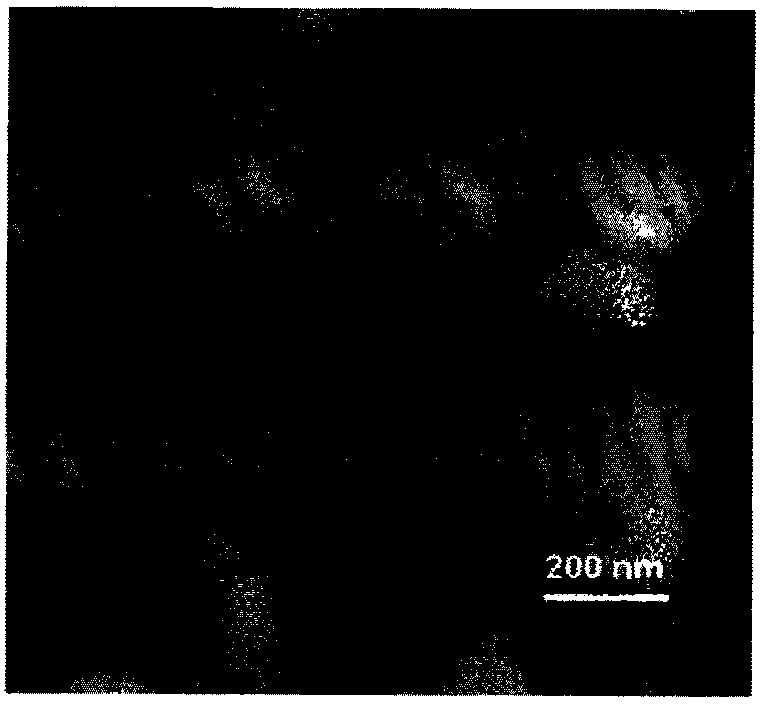
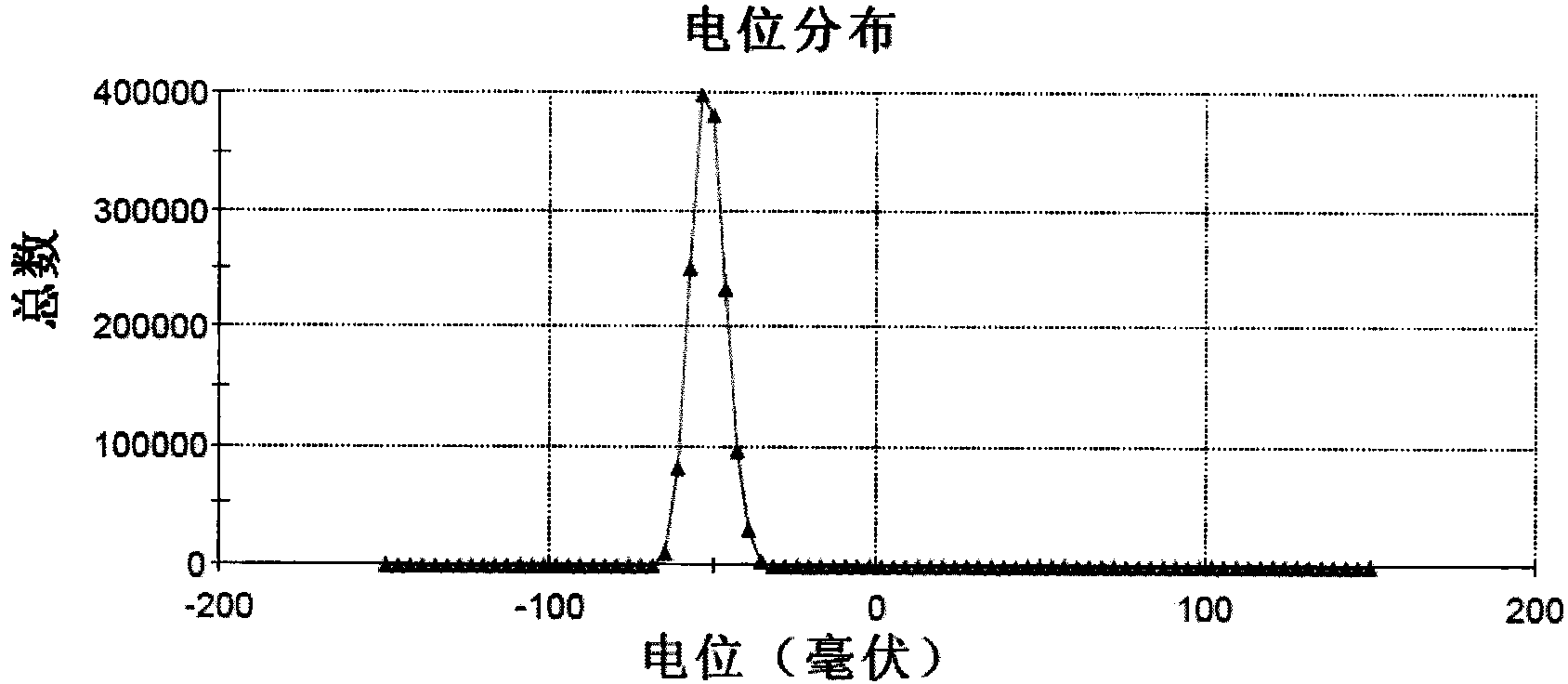
[0035] Example 1 Fullerenol C 60 (OH) 20 Synthesis
[0036] First, add 1ml of 40% tetrabutylammonium hydroxide solution (TBAH) and 2ml of sodium hydroxide solution (2.22g / ml) to 30ml of C 60(Sigma company) in toluene solution (1.5mg / ml), react 24h under vigorous stirring; Discard organic phase, and remove aqueous phase with vacuum rotary evaporator (RV06-ML 1-B, Germany); Wash sample with 50ml methanol, Methanol was removed by vacuum evaporation, and washed repeatedly for 3-5 times; after that, 10ml of deionized water was added to the sample, and the stirring was continued until the solution was transparent reddish-brown, then 20ml of deionized water was added, and the Sephadex G-25 chromatographic column was used to (Amersham Company, UK) purified the sample, and finally vacuum-dried to obtain fullerenol nanoparticles. Fullerenols with different numbers of hydroxyl groups can be obtained by adjusting the amount of sodium hydroxide added.
[0037] Utilize atomic ...
Embodiment 2
[0042] Example 2 Effect of Fullererol on Vitality of HEK293 Cells (ATCC )
[0043] 1. Preparation of complete medium containing fullerenol:
[0044] First, take an appropriate amount of fullererol obtained in Example 1, and prepare a 2 mM aqueous solution with sterile deionized water; then use complete DMEM (Dulbecco's modification of Eagle's medium) medium (containing 10% fetal bovine serum, Gibco Company) diluted fullererol aqueous solution to 500 μM, and carried out gradient dilution with complete medium to obtain a series of solutions with concentrations of 500, 100, 20, 4, 0.8, and 0.16 μM for future use.
[0045] 2. The effect of fullerenol on the viability of HEK293 cells:
[0046] First, HEK293 cells were digested with 0.25% trypsin, and after the digestion was terminated, centrifugation was performed at 1000 rpm for 5 min. Single-cell suspension was prepared in DMEM medium containing 10% fetal bovine serum (FBS), and 5 × 10 4 / ml concentration was inoculated in 9...
Embodiment 3
[0048] Example 3 Effect of fullererol on the viability of NIH3T3 cells (ATCC)
[0049] With reference to the method described in Example 2, investigate the effect of fullerenol nanoparticles on the viability of NIH3T3 cells, the results are shown in Figure 6 , similar to the results in Example 2, compared with the negative control group, the cell viability did not change significantly after the cells were treated with fullerenol nanoparticles for 48 hours, even in the highest dose group, the cell viability was still higher than 90%. Figure 7 Indicates the effect of fullerenol on the morphology of NIH3T3 cells, Figure 7 A is the normal control group, Figure 7 B represents the picture of the cells treated for 48 hours when the working concentration of fullerenol was 500 μM. It can be seen that the morphology of the cells in the test group is the same as that in the normal control group, showing a long spindle shape. All these indicate that fullerenol has good biocompatib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com